Climate Resilience and Adaptation in West African Oyster Fisheries: An Expert-Based Assessment of the Vulnerability of the Oyster Crassostrea tulipa to Climate Change
Abstract
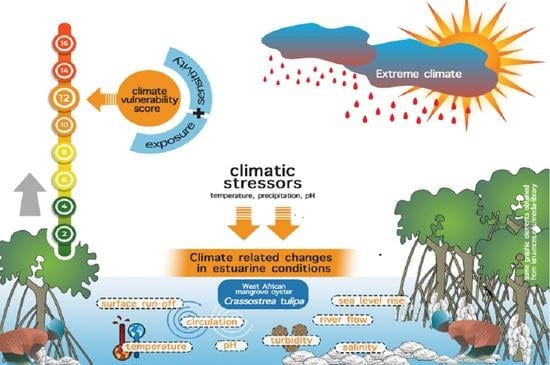
:1. Introduction
2. Literature Review
3. Methodology
3.1. Scoping, Planning, and Assessment Preparation
3.2. Scoring Process
3.3. Data Analysis
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lam, V.; Cheung, W.; Swartz, W.; Sumaila, U. Climate change impacts on fisheries in West Africa: Implications for economic, food and nutritional security. Afr. J. Mar. Sci. 2012, 34, 103–117. [Google Scholar] [CrossRef]
- Pörtner, H.-O.; Roberts, D.C.; Tignor, M.M.B.; Poloczanska, E.; Mintenbeck, K.; Alegría, A.; Craig, M.; Langsdorf, S.; Löschke, S.; Möller, V.; et al. Climate Change 2022: Impacts, Adaptation and Vulnerability; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- Atindana, S.A.; Fagbola, O.; Ajani, E.; Alhassan, E.H.; Ampofo-Yeboah, A. Coping with climate variability and non-climate stressors in the West African Oyster (Crassostrea tulipa) fishery in coastal Ghana. Marit. Stud. 2020, 19, 81–92. [Google Scholar] [CrossRef]
- Carney, J.A. Shellfish Collection in Senegambian Mangroves: A Female Knowledge System in a Priority Conservation Region. J. Ethnobiol. 2017, 37, 440–457. [Google Scholar] [CrossRef]
- Jimoh, A.A.; Lemomu, I.P. Shellfish resources in Nigeria. In Proceedings of the 25th Annual Conference of the Fisheries Society of Nigeria (FISON), Lagos, Nigeria, 25–29 October 2010. [Google Scholar]
- Monfort, M.C. The role of women in the seafood industry. Globefish Res. Programme 2015, 119, 67. [Google Scholar]
- Frankić, A. Healthy oysters for health oceans: A biomimetic approach. Med. Jadertina 2019, 49 (Suppl. S2), 22. [Google Scholar]
- Ahn, J.E.; Ronan, A.D. Development of a model to assess coastal ecosystem health using oysters as the indicator species. Estuar. Coast. Shelf Sci. 2020, 233, 106528. [Google Scholar] [CrossRef]
- Grabowski, J.H.; Peterson, C.H. Restoring oyster reefs to recover ecosystem services. Ecosyst. Eng. Plants Protists 2007, 4, 281–298. [Google Scholar] [CrossRef]
- Beck, M.W.; Brumbaugh, R.D.; Airoldi, L.; Carranza, A.; Coen, L.D.; Crawford, C.; Defeo, O.; Edgar, G.J.; Hancock, B.; Kay, M.C.; et al. Oyster Reefs at Risk and Recommendations for Conservation, Restoration, and Management. BioScience 2011, 61, 107–116. [Google Scholar] [CrossRef]
- Chuku, E.O.; Yankson, K.; Obodai, E.A.; Acheampong, E.; Boahemaa-Kobil, E.E. Effectiveness of different substrates for collecting wild spat of the oyster Crassostrea tulipa along the coast of Ghana. Aquac. Rep. 2020, 18, 100493. [Google Scholar] [CrossRef]
- Chuku, E.O. Promoting Oyster Culture in Ghana: Strategies for Optimising Seed Collection and Growth of Crassostrea tulipa (Lamarck, 1819) in Coastal Water Bodies; University of Cape Coast: Cape Coast, Ghana, 2019. [Google Scholar]
- Vilas, P.M.; Jadhav, A.C.; Dhavale, M.C.; Hasabnis, S.N.; Gaikwad, A.P.; Jadhav, P.R.; Ajit, P.S. Effect of cultural variability on mycellial growth of eleven mushroom isolates of Pleurotus spp. J. Pharmacogn. Phytochem. 2020, 9, 881–888. [Google Scholar]
- Obodai, E.; Yankson, Y.; Jnr, J.B. Seasonal changes in hydrographic factors and breeding in two population of Crassostea tulupa (Lamarch). Ghana J. Sci. 2009, 36, 47986. [Google Scholar] [CrossRef]
- Angell, C.L. The Biology and Culture of Tropical Oysters; ICLARM: Manila, Philippines, 1986; Volume 13. [Google Scholar]
- Yankson, K. Preliminary studies on the rearing of the West African Mangrove Oyster, Crassostrea tulipa, in the laboratory. Discov. Innov. 1990, 2, 45–51. [Google Scholar]
- Tomaru, Y.; Kawabata, Z.; Nakano, S. Mass mortality of the Japanese pearl oyster Pinctada fucata martensii in relation to water temperature, chlorophyll a and phytoplankton composition. Dis. Aquat. Org. 2001, 44, 61–68. [Google Scholar] [CrossRef]
- Fleury, P.; Goyard, E.; Mazurié, J.; Claude, S.; Bouget, J.; Langlade, A.; Le Coguic, Y. The assessing of Pacific oyster (Crassostrea gigas) rearing performances by the IFREMER/REMORA network: Method and first results (1993–1998) in Brittany (France). In Coastal Shellfish—A Sustainable Resource; Springer: Berlin/Heidelberg, Germany, 2001; pp. 195–208. [Google Scholar]
- Handisyde, N.; Telfer, T.; Ross, L.G. Vulnerability of aquaculture-related livelihoods to changing climate at the global scale. Fish Fish. 2017, 18, 466–488. [Google Scholar] [CrossRef]
- Hare, J.A.; Morrison, W.E.; Nelson, M.W.; Stachura, M.M.; Teeters, E.J.; Griffis, R.; Alexander, M.; Scott, J.D.; Alade, L.; Bell, R.J.; et al. A Vulnerability Assessment of Fish and Invertebrates to Climate Change on the Northeast U.S. Continental Shelf. PLoS ONE 2016, 11, e0146756. [Google Scholar] [CrossRef]
- Barsley, W.; De Young, C.; Brugère, C. Vulnerability assessment methodologies: An annotated bibliography for climate change and the fisheries and aquaculture sector. FAO Fish. Aquac. Circ. 2013, 1083, 1. [Google Scholar]
- Niang, I.; Oliver CAbdrabo, M.A.; Essel, A.; Lennard, C.; Padgham, J.; Urquhart, P. Climate Change 2014: Impacts, Adaptation and Vulnerability: Part B: Regional Aspects: Working Group II Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; pp. 1199–1266. [Google Scholar]
- Jafino, B.A.; Walsh, B.; Rozenberg, J.; Hallegatte, S. Revised Estimates of the Impact of Climate Change on Extreme Poverty by 2030. Policy Res. Work. Pap. 2020, 9417. [Google Scholar] [CrossRef]
- Watson, R.T.; Zinyowera, M.M.; Moss, R.H. The Regional Impacts of Climate Change: An Assessment of Vulnerability; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Allison, E.H. Effects of Climate Change on the Sustainability of Capture and Enhancement Fisheries Important to the Poor: Analysis of the Vulnerability and Adaptability of Fisherfolk Living in Poverty; Final Technical Report; Fisheries Management Science Programme. 2005. Available online: https://agris.fao.org/agris-search/search.do?recordID=GB2012105545 (accessed on 5 May 2022).
- Allison, E.H.; Perry, A.L.; Badjeck, M.-C.; Adger, W.N.; Brown, K.; Conway, D.; Halls, A.S.; Pilling, G.M.; Reynolds, J.D.; Andrew, N.L.; et al. Vulnerability of national economies to the impacts of climate change on fisheries. Fish Fish. 2009, 10, 173–196. [Google Scholar] [CrossRef]
- Alder, J.; Sumaila, U.R. Western Africa: A Fish Basket of Europe Past and Present. J. Environ. Dev. 2004, 13, 156–178. [Google Scholar] [CrossRef]
- Atta-Mills, J.; Alder, J.; Sumaila, U.R. The decline of a regional fishing nation: The case of Ghana and West Africa. Nat. Resour. Forum 2004, 28, 13–21. [Google Scholar] [CrossRef]
- Alexandratos, N.; Bruinsma, J.; Boedeker, G.; Schmidhuber, J.; Broca, S.S.; Shetty, P.; Ottaviani, P. World Agriculture towards 2030/2050: The 2012 Revision; ESA Working paper No. 12-03; FAO: Rome, Italy, 2012. [Google Scholar]
- Byerlee, D. World Development Report 2008: Agriculture for Development; World Bank: Washington, DC, USA, 2008. [Google Scholar]
- Cheung, W.W.L.; Lam, V.W.Y.; Sarmiento, J.L.; Kearney, K.; Watson, R.; Zeller, D.; Pauly, D. Large-scale redistribution of maximum fisheries catch potential in the global ocean under climate change. Glob. Chang. Biol. 2010, 16, 24–35. [Google Scholar] [CrossRef]
- Belhabib, D.; Sumaila, U.R.; Pauly, D. Feeding the poor: Contribution of West African fisheries to employment and food security. Ocean Coast. Manag. 2015, 111, 72–81. [Google Scholar] [CrossRef]
- Obayemi, I.M. Promoting women participation in fish culture enterprises—As a means of poverty alleviation in Nigeria. In Proceedings of the 19th Annual Conference of the Fisheries Society of Nigeria (FISON), Ilorin, Nigeria, 29 November–3 December 2004. [Google Scholar]
- Rothschild, B.; Ault, J.; Goulletquer, P.; Héral, M. Decline of the Chesapeake Bay oyster population: A century of habitat destruction and overfishing. Mar. Ecol. Prog. Ser. 1994, 111, 29–39. [Google Scholar] [CrossRef]
- Miller, A.W.; Reynolds, A.C.; Sobrino, C.; Riedel, G.F. Shellfish Face Uncertain Future in High CO2 World: Influence of Acidification on Oyster Larvae Calcification and Growth in Estuaries. PLoS ONE 2009, 4, e5661. [Google Scholar] [CrossRef]
- Doney, S.C.; Ruckelshaus, M.; Duffy, J.E.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate Change Impacts on Marine Ecosystems. Annu. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef]
- Sandison, E.E. The Effect of Salinity Fluctuations on the Life Cycle of Gryphaea gasar ((Adanson) Dautzenberg) in Lagos Harbour, Nigeria. J. Anim. Ecol. 1966, 35, 379. [Google Scholar] [CrossRef]
- Casas, S.; Filgueira, R.; Lavaud, R.; Comeau, L.; La Peyre, M.; La Peyre, J. Combined effects of temperature and salinity on the physiology of two geographically-distant eastern oyster populations. J. Exp. Mar. Biol. Ecol. 2018, 506, 82–90. [Google Scholar] [CrossRef]
- Pierce, R.H.; Henry, M.S. Harmful algal toxins of the Florida red tide (Karenia brevis): Natural chemical stressors in South Florida coastal ecosystems. Ecotoxicology 2008, 17, 623–631. [Google Scholar] [CrossRef]
- Matsuyama, Y.; Uchida, T.; Honjo, T. Effects of Harmful Dinoflagellates, Gymnodinium mikimotoi and Heterocapsa circularisquama, Red-tide on Filtering Rate of Bivalve Molluscs. Fish. Sci. 1999, 65, 248–253. [Google Scholar] [CrossRef]
- Bueno-Pardo, J.; Nobre, D.; Monteiro, J.N.; Sousa, P.M.; Costa, E.F.S.; Baptista, V.; Ovelheiro, A.; Vieira, V.M.N.C.S.; Chícharo, L.; Gaspar, M.; et al. Climate change vulnerability assessment of the main marine commercial fish and invertebrates of Portugal. Sci. Rep. 2021, 11, 2958. [Google Scholar] [CrossRef]
- Lotze, H.K.; Tittensor, D.P.; Bryndum-Buchholz, A.; Eddy, T.D.; Cheung, W.W.L.; Galbraith, E.D.; Barange, M.; Barrier, N.; Bianchi, D.; Blanchard, J.; et al. Global ensemble projections reveal trophic amplification of ocean biomass declines with climate change. Proc. Natl. Acad. Sci. USA 2019, 116, 12907–12912. [Google Scholar] [CrossRef]
- Bueno-Pardo, J.; Petitgas, P.; Kay, S.; Huret, M. Integration of bioenergetics in an individual-based model to hindcast anchovy dynamics in the Bay of Biscay. ICES J. Mar. Sci. 2019, 77, 655–667. [Google Scholar] [CrossRef]
- Williams, S.E.; Shoo, L.P.; Isaac, J.L.; Hoffmann, A.; Langham, G. Towards an Integrated Framework for Assessing the Vulnerability of Species to Climate Change. PLoS Biol. 2008, 6, e325-6. [Google Scholar] [CrossRef] [PubMed]
- Morrison, W.E.; Nelson, M.W.; Howard, J.F.; Teeters, E.J.; Hare, J.A.; Scott, J.D.; Alexander, M.A. Methodology for Assessing the Vulnerability of Marine Fish and Shellfish Species to a Changing Climate. 2015. Available online: https://repository.library.noaa.gov/view/noaa/5324 (accessed on 31 July 2022).
- Cotillon, S.E.; Tappan, G.G. Landscapes of West Africa: A Window on a Changing World; University of Arizona: Tucson, AZ, USA, 2016. [Google Scholar]
- Ajana, A. Fishery of the mangrove oyster, Crassostrea gasar, Adanson (1757), in the Lagos area, Nigeria. Aquaculture 1980, 21, 129–137. [Google Scholar] [CrossRef]
- Ilori, O.W.; Ajayi, V.O. Change Detection and Trend Analysis of Future Temperature and Rainfall over West Africa. Earth Syst. Environ. 2020, 4, 1–20. [Google Scholar] [CrossRef]
- Macadam, I.; Rowell, D.; Steptoe, H. Refining projections of future temperature change in West Africa. Clim. Res. 2020, 82, 1–14. [Google Scholar] [CrossRef]
- Almazroui, M.; Saeed, F.; Saeed, S.; Islam, M.N.; Ismail, M.; Klutse, N.A.B.; Siddiqui, M.H. Projected Change in Temperature and Precipitation over Africa from CMIP6. Earth Syst. Environ. 2020, 4, 455–475. [Google Scholar] [CrossRef]
- Druyan, L.M. Studies of 21st-century precipitation trends over West Africa. Int. J. Clim. 2010, 31, 1415–1424. [Google Scholar] [CrossRef]
- Wang, G.; Alo, C.A. Changes in Precipitation Seasonality in West Africa Predicted by RegCM3 and the Impact of Dynamic Vegetation Feedback. Int. J. Geophys. 2012, 2012, 597205. [Google Scholar] [CrossRef]
- Raj, J.; Bangalath, H.K.; Stenchikov, G. West African Monsoon: Current state and future projections in a high-resolution AGCM. Clim. Dyn. 2018, 52, 6441–6461. [Google Scholar] [CrossRef]
- Hartley, A.; Jones, R.; Janes, T. Projections of Change in Ecosystem Services under Climate Change. 2015. Available online: http://parcc.protectedplanet.net/system/comfy/cms/files/files/000/000/040/original/PARCC_Ecosystem_Services_FINAL_EN.pdf (accessed on 31 July 2022).
- Feely, R.; Doney, S.; Cooley, S. Ocean Acidification: Present Conditions and Future Changes in a High-CO2 World. Oceanography 2009, 22, 36–47. [Google Scholar] [CrossRef]
- Feely, R.A.; Orr, J.; Fabry, V.J.; Kleypas, J.A.; Sabine, C.L.; Langdon, C. Present and future changes in seawater chemistry due to ocean acidification. Geophys. Monogr. Ser. 2009, 183, 175–188. [Google Scholar] [CrossRef]
- Mori, N.; Shimura, T.; Yasuda, T.; Mase, H. Multi-model climate projections of ocean surface variables under different climate scenarios—Future change of waves, sea level and wind. Ocean. Eng. 2013, 71, 122–129. [Google Scholar] [CrossRef]
- De Bruijn, M.E.; van Dijk, J.W.M. Climate Change and Climatic Variability in West Africa; African Studies Centre: Leiden, The Netherlands, 2006; Available online: https://www.ascleiden.nl/Pdf/infosheet2.pdf (accessed on 5 May 2022).
- Sandison, E.E.; Hill, M.B. The Distribution of Balanus pallidus stutsburi Darwin, Gryphaea gasar ((Adanson) Dautzenberg), Mercierella enigmatica Fauvel and Hydroides uncinata (Philippi) in Relation to Salinity in Lagos Harbour and Adjacent Creeks. J. Anim. Ecol. 1966, 35, 235. [Google Scholar] [CrossRef]
- Dimock, R.V. An Examination of Physiological Variation in the American Oyster, Crassostrea virginica; Florida State University: Tallahassee, FL, USA, 1967. [Google Scholar]
- Loosanoff, V.L. Some Aspects of Behavior of Oysters at Different Temperatures. Biol. Bull. 1958, 114, 57–70. [Google Scholar] [CrossRef]
- Hand, S.C.; Stickle, W.B. Effects of tidal fluctuations of salinity on pericardial fluid composition of the American oyster Crassostrea virginica. Mar. Biol. 1977, 42, 259–271. [Google Scholar] [CrossRef]
- Gazeau, F.; Quiblier, C.; Jansen, J.M.; Gattuso, J.-P.; Middelburg, J.J.; Heip, C.H.R. Impact of elevated CO2 on shellfish calcification. Geophys. Res. Lett. 2007, 34, 7. [Google Scholar] [CrossRef]
- Mahu, E.; Nyarko, E.; Hulme, S.; Coale, K.H. Distribution and enrichment of trace metals in marine sediments from the Eastern Equatorial Atlantic, off the Coast of Ghana in the Gulf of Guinea. Mar. Pollut. Bull. 2015, 98, 301–307. [Google Scholar] [CrossRef]
- Mahu, E.; Nyarko, E.; Hulme, S.; Swarzenski, P.; Asiedu, D.K.; Coale, K.H. Geochronology and historical deposition of trace metals in three tropical estuaries in the Gulf of Guinea. Estuar. Coast. Shelf Sci. 2016, 177, 31–40. [Google Scholar] [CrossRef]
- Adika, S.A.; Mahu, E.; Crane, R.; Marchant, R.; Montford, J.; Folorunsho, R.; Gordon, C. Microplastic ingestion by pelagic and demersal fish species from the Eastern Central Atlantic Ocean, off the Coast of Ghana. Mar. Pollut. Bull. 2020, 153, 110998. [Google Scholar] [CrossRef]
- Go, J.; Deutscher, A.T.; Spiers, Z.B.; Dahle, K.; Kirkland, P.D.; Jenkins, C. Mass mortalities of unknown aetiology in Pacific oysters Crassostrea gigas in Port Stephens, New South Wales, Australia. Dis. Aquat. Org. 2017, 125, 227–242. [Google Scholar] [CrossRef] [PubMed]
- King, W.L.; Jenkins, C.; Go, J.; Siboni, N.; Seymour, J.R.; Labbate, M. Characterisation of the Pacific Oyster Microbiome during a Summer Mortality Event. Microb. Ecol. 2018, 77, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Ajonina, G.; Diamé, A.; Kairo, J. Current status and conservation of mangroves in Africa: An overview. World Rainfor. Mov. Bull. 2008, 133, 1–6. [Google Scholar]








| Expert Code | Gender | Shellfish (Fishery, Biology, Ecology, Aquaculture | Climate Change (Science, Socio-Ecology, Development | Years of Experience |
|---|---|---|---|---|
| E1 | Male | Yes | Yes | 8 |
| E2 | Female | Yes | Yes | 5 |
| E3 | Female | Yes | Yes | 13 |
| E4 | Male | Yes | Yes | 10 |
| E5 | Female | Yes | Yes | 15 |
| E6 | Male | Yes | Yes | 30 |
| E7 | Female | Yes | Yes | 6 |
| E8 | Male | Yes | Yes | 6 |
| E9 | Male | Yes | Yes | 30 |
| Exposure/Sensitivity Attributes | Requirements |
|---|---|
| Temperature | Thrives in a temperature range of 18–33 °C. Temperatures below 10 °C and above 35 °C may be lethal. |
| Salinity | Thrives in a salinity range of 4 ppt and 50‰. Salinities below 4 ppt and above 50‰ are potentially detrimental to growth and survival. Prolonged exposure to a salinity of 0 ppt causes mortalities. |
| pH | Thrives in a pH range of 6–8.5. Extremely low and high pH has significant detrimental effects on shell development. |
| Dissolved Oxygen | Thrives in low concentrations of DO up to about 1 mg/L. |
| Water Flow/Residual Current | Thrive within 0.5 m/s to 1.5 m/s. Stagnant water (0 m/s will disrupt filter feeding and high-speed flowing water (above 1.5 m/s) will not allow spats to settle on suitable substrate. |
| Length of submergence in water and exposure to air at low tide | Survives up to 36 h out of the marine environment whilst exposure periods beyond 3 days lead to mortality. Growth is retarded in long periods of exposure as feeding halts during exposure. |
| Turbidity | Excess silt smothers the gills, hampers the flow of food during filter feeding and leads to mortalities. |
| Habitat Specificity | A habitat specialist adapted to the near shore intertidal areas where its prime habitat is the stilt roots of red mangroves. The pediveliger larvae fasten to available hard stable substrates when settling. Thrives well in brackish environments with almost no survival in freshwater environments. |
| Prey specificity | Has a specific prey item. The preferred diet is Phytoplankton. |
| Stock size and status | Depends on environmental conditions for spat, availability of substrates for attachment and the ability of parent stocks to reproduce. Stock size has declined in some ecosystems; more data needed to quantify the rate of decline. |
| Adult mobility | The adult form has no active movement. Movement may be passive floating substrates. |
| Spawning cycle | Spawn all-year round. Spawn depending on conducive environmental conditions. |
| Reproductive strategy (including any complexity) | Broadcast fertilization: Males and female gametes are released into the open water environment and fertilization occurs at random. The species is highly proliferous with several thousands of egg cells released per individual during spawning. It exhibits protandric sequential hermaphroditism, i.e., the matured form differentiates first as male, then later as female in an individual organism. |
| Early life history survival and settlement | The eggs are fertilized in the water and within hours, develop into microscopic larvae that drift in the plankton, guided by the tides and currents. For up to 4 weeks, the larvae drifts in the coastal and estuaries waters during which they develop transparent shells and a retractable foot. Survival rates during this phase of the life cycle may be very low, however, the millions of eggs and sperms released in one season ensures successful settlement of most larvae. The larvae (about 0.3 mm in length) settle on a clean, hard substrate (surface) using the foot to crawl around to find a suitable site for permanent settlement. Once attached, the foot is reabsorbed into the body of the larvae. |
| Dispersal of early life stages | Dispersal of larvae from parent stocks by water currents through swimming is aided by ciliated velum. Larval settlement stage is about 4 weeks. |
| Population growth rate | Maturity is reached in roughly between 3–12 months with growth rates varying depending on local conditions. No knowledge of data on the population growth rate. |
| Other stressors | Pollution (plastics, heavy metals, PCBs, sediments, etc.), River flow modifications, mangrove deforestation, fouling organisms, predators, parasites, competition (space, food, etc.), natural disasters (storms, etc.), over exploitation of minerals in oyster beds put pressures on the oysters, invasive species. |
| Climate Exposure | Source | Model | Region |
|---|---|---|---|
| Temperature | [48] | CMIP-5 | West Africa |
| Temperature | [49] | CMIP-5 | West Africa |
| Temperature | [50] | CMIP-6 | Africa |
| Precipitation | [50] | CMIP-6 | Africa |
| Precipitation | [51] | AOGCM | West Africa |
| Precipitation | [52] | RegCM3 | West Africa |
| Precipitation | [53] | HiRAM SM2M | West Africa |
| Surface run-off | [54] | AIM, MINICAM, noLUC | West Africa |
| Ocean Acidification | [55] | CCSM3, GLODAP | Global |
| Ocean Acidification | [56] | OCIMP-2 | Global |
| Ocean circulation | [57] | CMIP-3/SRES | Global |
| Sea level rise | [57] | CMIP-3/SRES | Global |
| Data Quality Score | Description |
|---|---|
| 3 | Adequate Data. The score is based on data which have been observed, modeled or empirically measured for the C. tulipa and comes from a reputable source |
| 2 | Limited Data. The score is based on data which has a higher degree of uncertainty. The data used to score the attribute may be based on related or similar species, come from outside the study area, or the reliability of the source may be limited. |
| 1 | Expert Judgement. The attribute score reflects the expert judgement of the reviewer and is based on their general knowledge of the C. tulipa and its role in the ecosystem |
| 0 | No data. No information to base an attribute score on. Very little is known about the C. tulipa or related and there is no basis for forming an expert opinion. |
| Overall Sensitivity or Exposure Score | Numeric Score | Logic Rule |
|---|---|---|
| Very High | 4 | 3 or more attributes or factor means ≥3.5 |
| High | 3 | 2 or more attributes or factor means ≥3.0 |
| Moderate | 2 | 2 or more attributes or factor means ≥2.5 |
| Low | 1 | Less than 2 or more attribute or factor ≥2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahu, E.; Sanko, S.; Kamara, A.; Chuku, E.O.; Effah, E.; Sohou, Z.; Zounon, Y.; Akinjogunla, V.; Akinnigbagbe, R.O.; Diadhiou, H.D.; et al. Climate Resilience and Adaptation in West African Oyster Fisheries: An Expert-Based Assessment of the Vulnerability of the Oyster Crassostrea tulipa to Climate Change. Fishes 2022, 7, 205. https://doi.org/10.3390/fishes7040205
Mahu E, Sanko S, Kamara A, Chuku EO, Effah E, Sohou Z, Zounon Y, Akinjogunla V, Akinnigbagbe RO, Diadhiou HD, et al. Climate Resilience and Adaptation in West African Oyster Fisheries: An Expert-Based Assessment of the Vulnerability of the Oyster Crassostrea tulipa to Climate Change. Fishes. 2022; 7(4):205. https://doi.org/10.3390/fishes7040205
Chicago/Turabian StyleMahu, Edem, Salieu Sanko, Allieubakarr Kamara, Ernest Obeng Chuku, Elizabeth Effah, Zacharie Sohou, Yaovi Zounon, Victoria Akinjogunla, Ruth Oluwatoyin Akinnigbagbe, Hamet Diaw Diadhiou, and et al. 2022. "Climate Resilience and Adaptation in West African Oyster Fisheries: An Expert-Based Assessment of the Vulnerability of the Oyster Crassostrea tulipa to Climate Change" Fishes 7, no. 4: 205. https://doi.org/10.3390/fishes7040205