Comparison of Overall Sensitivity and Specificity across Different Newborn Screening Algorithms for Congenital Cytomegalovirus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Assessment of Validity
2.2. Screening Algorithms
2.3. Identification of Hypothetical cCMV Cases Using Algorithm
3. Results
3.1. Validity Measures
3.2. Predicted Hypothetical cCMV Cases Using Algorithm
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, M.; Shorman, M. Cytomegalovirus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Centers for Disease Control and Prevention. About Cytomegalovirus (CMV). Available online: https://www.cdc.gov/cmv/overview.html (accessed on 21 February 2023).
- Louten, J. Virus Transmission and Epidemiology. In Essential Human Virology; Academic Press: Cambridge, MA, USA, 2016; pp. 71–92. [Google Scholar]
- Pass, R.F.; Anderson, B. Mother-to-Child Transmission of Cytomegalovirus and Prevention of Congenital Infection. J. Pediatr. Infect. Dis. Soc. 2014, 3 (Suppl. 1), S2–S6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. Babies Born with Congenital Cytomegalovirus (CMV). Available online: https://www.cdc.gov/cmv/congenital-infection.html (accessed on 9 August 2022).
- Fowler, K.B.; McCollister, F.P.; Sabo, D.L.; Shoup, A.G.; Owen, K.E.; Woodruff, J.L.; Cox, E.; Mohamed, L.S.; Choo, D.I.; Boppana, S.B. A Targeted Approach for Congenital Cytomegalovirus Screening within Newborn Hearing Screening. Pediatrics 2017, 139, e20162128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenneson, A.; Cannon, M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 2007, 17, 253–276. [Google Scholar] [CrossRef]
- Manicklal, S.; Emery, V.C.; Lazzarotto, T.; Boppana, S.B.; Gupta, R.K. The “silent” global burden of congenital cytomegalovirus. Clin. Microbiol. Rev. 2013, 26, 86–102. [Google Scholar] [CrossRef] [Green Version]
- Gantt, S.; Bitnun, A.; Renaud, C.; Kakkar, F.; Vaudry, W. Diagnosis and management of infants with congenital cytomegalovirus infection. Paediatr. Child Health 2017, 22, 72–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesch, M.H.; Schleiss, M.R. Emerging Concepts in Congenital Cytomegalovirus. Pediatrics 2022, 150, e2021055896. [Google Scholar] [CrossRef]
- Dietrich, M.L.; Schieffelin, J.S. Congenital Cytomegalovirus Infection. Ochsner J. 2019, 19, 123–130. [Google Scholar] [CrossRef]
- Boppana, S.B.; Ross, S.A.; Fowler, K.B. Congenital cytomegalovirus infection: Clinical outcome. Clin. Infect. Dis. 2013, 57, S178–S181. [Google Scholar] [CrossRef] [Green Version]
- Kabani, N.; Ross, S.A. Congenital cytomegalovirus infection. J. Infect. Dis. 2020, 221, S9–S14. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.M.; Mitchell, R.; Knight, J.A.; Mazzulli, T.; Relton, C.; Khodayari Moez, E.; Hung, R.J. Early-childhood cytomegalovirus infection and children’s neurocognitive development. Int. J. Epidemiol. 2021, 50, 538–549. [Google Scholar] [CrossRef]
- Rawlinson, W.D.; Boppana, S.B.; Fowler, K.B.; Kimberlin, D.W.; Lazzarotto, T.; Alain, S.; Daly, K.; Doutre, S.; Gibson, L.; Giles, M.L.; et al. Congenital cytomegalovirus infection in pregnancy and the neonate: Consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect. Dis. 2017, 17, e177–e188. [Google Scholar] [CrossRef]
- Cannon, M.J.; Griffiths, P.D.; Aston, V.; Rawlinson, W.D. Universal newborn screening for congenital CMV infection: What is the evidence of potential benefit? Rev. Med. Virol. 2014, 24, 291–307. [Google Scholar] [CrossRef] [Green Version]
- Dollard, S.C.; Dreon, M.; Hernandez-Alvarado, N.; Amin, M.M.; Wong, P.; Lanzieri, T.M.; Osterholm, E.A.; Sidebottom, A.; Rosendahl, S.; McCann, M.T.; et al. Sensitivity of Dried Blood Spot Testing for Detection of Congenital Cytomegalovirus Infection. JAMA Pediatr. 2021, 175, e205441. [Google Scholar] [CrossRef]
- Rawlinson, W.D.; Palasanthiran, P.; Hall, B.; Al Yazidi, L.; Cannon, M.J.; Cottier, C.; van Zuylen, W.J.; Wilkinson, M. Neonates with congenital Cytomegalovirus and hearing loss identified via the universal newborn hearing screening program. J. Clin. Virol. 2018, 102, 110–115. [Google Scholar] [CrossRef]
- National Institute on Deafness and Other Communication Disorders (NIDCD). Your Baby’s Hearing Screening and Next Steps. Available online: https://www.nidcd.nih.gov/health/your-babys-hearing-screening-and-next-steps (accessed on 31 October 2022).
- Minnesota Department of Health. Congenital Cytomegalovirus Approved for Addition to Newborn Screening Panel. Available online: https://www.health.state.mn.us/index.html (accessed on 5 July 2022).
- Newborn Screening Ontario. Hearing Loss Risk Factor Screening. Available online: https://www.newbornscreening.on.ca/en/page/congenital-cytomegalovirus (accessed on 23 June 2022).
- CMV Canada. Saskatchewan Screens For cCMV! Available online: https://cmvcanada.com/saskatchewan-screens-for-ccmv/ (accessed on 23 June 2022).
- The Office of Governor Ned Lamont. Governor Lamont Reaches Budget Agreement with Legislative Leaders That Includes the Largest Income Tax Cut in Connecticut History. Available online: https://portal.ct.gov/Office-of-the-Governor/News/Press-Releases/2023/06-2023/Governor-Lamont-Reaches-Budget-Agreement-With-Legislative-Leaders (accessed on 13 June 2023).
- National CMV Foundation. Newborn Screening. Available online: https://www.nationalcmv.org/overview/newborn-screening (accessed on 28 July 2022).
- Pellegrinelli, L.; Alberti, L.; Pariani, E.; Barbi, M.; Binda, S. Diagnosing congenital Cytomegalovirus infection: Don’t get rid of dried blood spots. BMC Infect. Dis. 2020, 20, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinstein, S.; Obuchowski, N.A.; Lieber, M.L. Clinical Evaluation of Diagnostic Tests. Am. J. Roentgenol. 2005, 184, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.C.; Nance, W.E. Newborn hearing screening—A silent revolution. N. Engl. J. Med. 2006, 354, 2151–2164. [Google Scholar] [CrossRef] [PubMed]
- Mehra, S.; Eavey, R.D.; Keamy, D.G., Jr. The epidemiology of hearing impairment in the United States: Newborns, children, and adolescents. Otolaryngol. Head Neck Surg. 2009, 140, 461–472. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Congenital CMV and Hearing Loss. Available online: https://www.cdc.gov/cmv/hearing-loss.html (accessed on 8 June 2022).
- Maltezou, P.G.; Kourlaba, G.; Kourkouni, Ε.; Luck, S.; Blázquez-Gamero, D.; Ville, Y.; Lilleri, D.; Dimopoulou, D.; Karalexi, M.; Papaevangelou, V. Maternal type of CMV infection and sequelae in infants with congenital CMV: Systematic review and meta-analysis. J. Clin. Virol. 2020, 129, 104518. [Google Scholar] [CrossRef]
- Tzanakakis, M.G.; Chimona, T.S.; Apazidou, E.; Giannakopoulou, C.; Velegrakis, G.A.; Papadakis, C.E. Transitory evoked otoacoustic emission (TEOAE) and distortion product otoacoustic emission (DPOAE) outcomes from a three-stage newborn hearing screening protocol. Hippokratia 2016, 20, 104–109. [Google Scholar]
- Kuki, S.; Chadha, S.; Dhingra, S.; Gulati, A. The role of current audiological tests in the early diagnosis of hearing impairment in infant. Indian J. Otolaryngol. Head Neck Surg. 2013, 65, 244–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimberlin, D.W.; Jester, P.M.; Sanchez, P.J.; Ahmed, A.; Arav-Boger, R.; Michaels, M.G.; Ashouri, N.; Englund, J.A.; Estrada, B.; Jacobs, R.F.; et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. N. Engl. J. Med. 2015, 372, 933–943. [Google Scholar] [CrossRef] [Green Version]
- Cannon, M.J.; Levis, D.M.; McBride, H.; Watson, D.; Rheaume, C.; Hall, M.A.K.; Lanzieri, T.M.; Demmler-Harrison, G. Family Perceptions of Newborn Cytomegalovirus Screening: A Qualitative Study. Int. J. Neonatal Screen. 2021, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- National CMV Foundation. Advocacy in Action. Available online: https://www.nationalcmv.org/about-us/advocacy (accessed on 21 February 2023).
- Su, X.; Carlson, B.F.; Wang, X.; Li, X.; Zhang, Y.; Montgomery, J.P.; Ding, Y.; Wagner, A.L.; Gillespie, B.; Boulton, M.L. Dried blood spots: An evaluation of utility in the field. J. Infect. Public Health 2018, 11, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Baby’s First Test. The Recommended Uniform Screening Panel. Available online: https://www.babysfirsttest.org/newborn-screening/the-recommended-uniform-screening-panel (accessed on 21 February 2023).



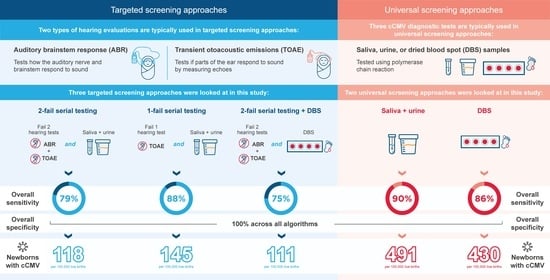
| Targeted Screening Algorithms | Universal Screening Algorithms | ||||
|---|---|---|---|---|---|
| Two-Fail Serial Testing 1 | One-Fail Serial Testing 2 | Two-Fail Serial Testing + DBS 3 | Universal Screening (Saliva + Urine PCR) | Universal Screening (DBS) | |
| Validity measure | |||||
| OSn (%) | 78.8 | 87.6 | 75.2 | 90.2 | 86.0 |
| OSp (%) | 100 | 100 | 100 | 100 | 100 |
| Cases of cCMV infection 4 | |||||
| Cases identified | 118 | 145 | 111 | 491 | 430 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schleiss, M.R.; Panther, L.; Basnet, S.; Workneh, M.; Diaz-Decaro, J. Comparison of Overall Sensitivity and Specificity across Different Newborn Screening Algorithms for Congenital Cytomegalovirus. Int. J. Neonatal Screen. 2023, 9, 33. https://doi.org/10.3390/ijns9020033
Schleiss MR, Panther L, Basnet S, Workneh M, Diaz-Decaro J. Comparison of Overall Sensitivity and Specificity across Different Newborn Screening Algorithms for Congenital Cytomegalovirus. International Journal of Neonatal Screening. 2023; 9(2):33. https://doi.org/10.3390/ijns9020033
Chicago/Turabian StyleSchleiss, Mark R., Lori Panther, Sandeep Basnet, Meklit Workneh, and John Diaz-Decaro. 2023. "Comparison of Overall Sensitivity and Specificity across Different Newborn Screening Algorithms for Congenital Cytomegalovirus" International Journal of Neonatal Screening 9, no. 2: 33. https://doi.org/10.3390/ijns9020033