Journal Description
International Journal of Neonatal Screening
International Journal of Neonatal Screening
is an international, peer-reviewed, open access journal on neonatal screening and neonatal medicine published quarterly online by MDPI. The journal is owned by the International Society for Neonatal Screening (ISNS). The International Society for Neonatal Screening (ISNS), German Society for Neonatal Screening (DGNS), the Japanese Society for Neonatal Screening (JSNS), the Association of Public Health Laboratories (APHL) and the UK Newborn Screening Laboratory Network (UKNSLN) are affiliated with IJNS and their members receive discounts on the article processing charges.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, ESCI (Web of Science), PubMed, PMC, Embase, and other databases.
- Journal Rank: CiteScore - Q1 (Pediatrics, Perinatology and Child Health)
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 21.3 days after submission; acceptance to publication is undertaken in 3.6 days (median values for papers published in this journal in the second half of 2023).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
Impact Factor:
3.5 (2022)
Latest Articles
Long-Term Follow-Up Cares and Check Initiative: A Program to Advance Long-Term Follow-Up in Newborns Identified with a Disease through Newborn Screening
Int. J. Neonatal Screen. 2024, 10(2), 34; https://doi.org/10.3390/ijns10020034 - 18 Apr 2024
Abstract
In the United States and around the world, newborns are screened on a population basis for conditions benefiting from pre-symptomatic diagnosis and treatment. The number of screened conditions continues to expand as novel technologies for screening, diagnosing, treating, and managing disease are discovered.
[...] Read more.
In the United States and around the world, newborns are screened on a population basis for conditions benefiting from pre-symptomatic diagnosis and treatment. The number of screened conditions continues to expand as novel technologies for screening, diagnosing, treating, and managing disease are discovered. While screening all newborns facilitates early diagnosis and treatment, most screened conditions are treatable but not curable. Patients identified by newborn screening often require lifelong medical management and community support to achieve the best possible outcome. To advance the long-term follow-up of infants identified through newborn screening (NBS), the Long-Term Follow-up Cares and Check Initiative (LTFU-Cares and Check) designed, implemented, and evaluated a system of longitudinal data collection and annual reporting engaging parents, clinical providers, and state NBS programs. The LTFU-Cares and Check focused on newborns identified with spinal muscular atrophy (SMA) through NBS and the longitudinal health information prioritized by parents and families. Pediatric neurologists who care for newborns with SMA entered annual data, and data tracking and visualization tools were delivered to state NBS programs with a participating clinical center. In this publication, we report on the development, use of, and preliminary results from the LTFU-Cares and Check Initiative, which was designed as a comprehensive model of LTFU. We also propose next steps for achieving the goal of a national system of LTFU for individuals with identified conditions by meaningfully engaging public health agencies, clinicians, parents, families, and communities.
Full article
(This article belongs to the Special Issue A Lifespan Approach to Health and Well-Being Leveraging Neonatal Screening: Efforts in Advocacy, Academia, Research, and Clinical Care)
►
Show Figures
Open AccessCommentary
An Opportunity to Fill a Gap for Newborn Screening of Neurodevelopmental Disorders
by
Wendy K. Chung, Stephen M. Kanne and Zhanzhi Hu
Int. J. Neonatal Screen. 2024, 10(2), 33; https://doi.org/10.3390/ijns10020033 - 16 Apr 2024
Abstract
Screening newborns using genome sequencing is being explored due to its potential to expand the list of conditions that can be screened. Previously, we proposed the need for large-scale pilot studies to assess the feasibility of screening highly penetrant genetic neurodevelopmental disorders. Here,
[...] Read more.
Screening newborns using genome sequencing is being explored due to its potential to expand the list of conditions that can be screened. Previously, we proposed the need for large-scale pilot studies to assess the feasibility of screening highly penetrant genetic neurodevelopmental disorders. Here, we discuss the initial experience from the GUARDIAN study and the systemic gaps in clinical services that were identified in the early stages of the pilot study.
Full article
(This article belongs to the Special Issue A Lifespan Approach to Health and Well-Being Leveraging Neonatal Screening: Efforts in Advocacy, Academia, Research, and Clinical Care)
Open AccessArticle
Assessing the Benefits and Harms Associated with Early Diagnosis from the Perspective of Parents with Multiple Children Diagnosed with Duchenne Muscular Dystrophy
by
Oindrila Bhattacharyya, Nicola B. Campoamor, Niki Armstrong, Megan Freed, Rachel Schrader, Norah L. Crossnohere and John F. P. Bridges
Int. J. Neonatal Screen. 2024, 10(2), 32; https://doi.org/10.3390/ijns10020032 - 15 Apr 2024
Abstract
Duchenne muscular dystrophy (DMD) is a rare neuromuscular disorder diagnosed in childhood. Limited newborn screening in the US often delays diagnosis. With multiple FDA-approved therapies, early diagnosis is crucial for timely treatment but may entail other benefits and harms. Using a community-based survey,
[...] Read more.
Duchenne muscular dystrophy (DMD) is a rare neuromuscular disorder diagnosed in childhood. Limited newborn screening in the US often delays diagnosis. With multiple FDA-approved therapies, early diagnosis is crucial for timely treatment but may entail other benefits and harms. Using a community-based survey, we explored how parents of siblings with DMD perceived early diagnosis of one child due to a prior child’s diagnosis. We assessed parents’ viewpoints across domains including diagnostic journey, treatment initiatives, service access, preparedness, parenting, emotional impact, and caregiving experience. We analyzed closed-ended responses on a −1.0 to +1.0 scale to measure the degree of harm or benefit parents perceived and analyzed open-ended responses thematically. A total of 45 parents completed the survey, with an average age of 43.5 years and 20.0% identifying as non-white. Younger siblings were diagnosed 2 years earlier on average (p < 0.001). Overall, parents viewed early diagnosis positively (mean: 0.39), particularly regarding school preparedness (+0.79), support services (+0.78), treatment evaluation (+0.68), and avoiding diagnostic odyssey (+0.67). Increased worry was a common downside (−0.40). Open-ended responses highlighted improved outlook and health management alongside heightened emotional distress and treatment burdens. These findings address gaps in the evidence by documenting the effectiveness of early screening and diagnosis of DMD using sibling data.
Full article
(This article belongs to the Special Issue A Lifespan Approach to Health and Well-Being Leveraging Neonatal Screening: Efforts in Advocacy, Academia, Research, and Clinical Care)
►▼
Show Figures
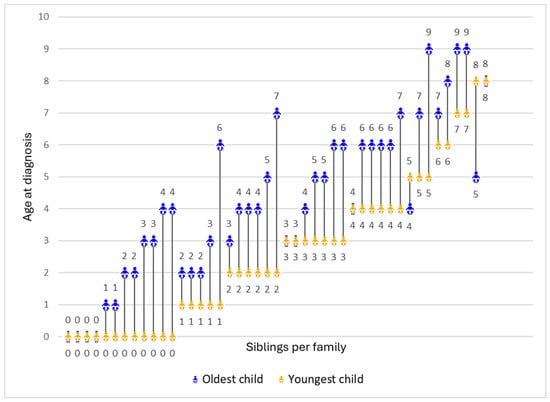
Figure 1
Open AccessArticle
International Perspectives of Extended Genetic Sequencing When Used as Part of Newborn Screening to Identify Cystic Fibrosis
by
Corinna C. A. Clark, Pru Holder, Felicity K. Boardman, Louise Moody, Jacqui Cowlard, Lorna Allen, Claire Walter, James R. Bonham and Jane Chudleigh
Int. J. Neonatal Screen. 2024, 10(2), 31; https://doi.org/10.3390/ijns10020031 - 08 Apr 2024
Abstract
There is increasing interest in using extended genetic sequencing (EGS) in newborn screening (NBS) for cystic fibrosis (CF). How this is implemented will change the number of children being given an uncertain outcome of CRMS/CFSPID (cystic fibrosis transmembrane conductance regulator (CFTR)-related metabolic syndrome/CF
[...] Read more.
There is increasing interest in using extended genetic sequencing (EGS) in newborn screening (NBS) for cystic fibrosis (CF). How this is implemented will change the number of children being given an uncertain outcome of CRMS/CFSPID (cystic fibrosis transmembrane conductance regulator (CFTR)-related metabolic syndrome/CF Screen Positive Inconclusive Diagnosis), probable carrier results, and the number of missed CF diagnoses. An international survey of CF health professionals was used to gather views on two approaches to EGS—specific (may reduce detection of CRMS/CFSID but miss some CF cases) versus sensitive (may increase detection of CRMS/CFSPID but avoid missing more CF cases). Health professionals acknowledged the anxiety caused to parents (and health professionals) from the uncertainty surrounding the prognosis and management of CRMS/CFSPID. However, most preferred the sensitive approach, as overall, identifying more cases of CRMS/CFSPID was viewed as less physically and psychologically damaging than a missed case of CF. The importance of early diagnosis and treatment for CF to ensure better health outcomes and reducing diagnostic odysseys for parents were highlighted. A potential benefit to identifying more children with CRMS/CFSPID included increasing knowledge to obtain a better understanding of how these children should best be managed in the future.
Full article
Open AccessConference Report
Newborn Screening Today and Tomorrow: A Brief Report from the International Primary Immunodeficiencies Congress
by
Leire Solis, Samya Van Coillie, James R. Bonham, Fabian Hauck, Lennart Hammarström, Frank J. T. Staal, Bruce Lim, Martine Pergent and Johan Prévot
Int. J. Neonatal Screen. 2024, 10(2), 30; https://doi.org/10.3390/ijns10020030 - 05 Apr 2024
Abstract
This article presents the report of the session on “Newborn Screening for Primary Immunodeficiencies—Now What?” organised during the International Primary Immunodeficiency Congress (IPIC) held in November 2023. This clinical conference was organised by the International Patient Organisation for Primary Immunodeficiencies (IPOPI), the global
[...] Read more.
This article presents the report of the session on “Newborn Screening for Primary Immunodeficiencies—Now What?” organised during the International Primary Immunodeficiency Congress (IPIC) held in November 2023. This clinical conference was organised by the International Patient Organisation for Primary Immunodeficiencies (IPOPI), the global patient organisation advocating for primary immunodeficiencies (PIDs) in patients. The session aimed at exploring the advances in newborn screening (NBS) for severe combined immunodeficiency, starting with the common practice and inserting the discussion into the wider perspective of genomics whilst taking into consideration the ethical aspects of screening as well as incorporating families and the public into the discussions, so as to ensure that NBS for treatable rare disorders continues to be one of the major public health advances of the 20th century.
Full article
Open AccessArticle
Management and Outcomes of Very Long-Chain Acyl-CoA Dehydrogenase Deficiency (VLCAD Deficiency): A Retrospective Chart Review
by
Maria Al Bandari, Laura Nagy, Vivian Cruz, Stacy Hewson, Alomgir Hossain and Michal Inbar-Feigenberg
Int. J. Neonatal Screen. 2024, 10(2), 29; https://doi.org/10.3390/ijns10020029 - 30 Mar 2024
Abstract
Very long-chain acyl-CoA dehydrogenase (VLCAD) deficiency is a rare genetic condition affecting the mitochondrial beta-oxidation of long-chain fatty acids. This study reports on the clinical outcomes of patients diagnosed by newborn screening with VLCAD deficiency comparing metabolic parameters, enzyme activities, molecular results, and
[...] Read more.
Very long-chain acyl-CoA dehydrogenase (VLCAD) deficiency is a rare genetic condition affecting the mitochondrial beta-oxidation of long-chain fatty acids. This study reports on the clinical outcomes of patients diagnosed by newborn screening with VLCAD deficiency comparing metabolic parameters, enzyme activities, molecular results, and clinical management. It is a single-center retrospective chart review of VLCAD deficiency patients who met the inclusion criteria between January 2002 and February 2020. The study included 12 patients, 7 of whom had an enzyme activity of more than 10%, and 5 patients had an enzyme activity of less than 10%. The Pearson correlation between enzyme activity and the C14:1 level at newborn screening showed a p-value of 0.0003, and the correlation between enzyme activity and the C14:1 level at diagnosis had a p-value of 0.0295. There was no clear correlation between the number of documented admissions and the enzyme activity level. Patients who had a high C14:1 value at diagnosis were started on a diet with a lower percentage of energy from long-chain triglycerides. The C14:1 result at diagnosis is the value that has been guiding our initial clinical management in asymptomatic diagnosed newborns. However, the newborn screening C14:1 value is the most sensitive predictor of low enzyme activity and may help guide dietary management.
Full article
Open AccessArticle
Newborn Screening for Inborn Errors of Metabolism by Next-Generation Sequencing Combined with Tandem Mass Spectrometry
by
Chengfang Tang, Lixin Li, Ting Chen, Yulin Li, Bo Zhu, Yinhong Zhang, Yifan Yin, Xiulian Liu, Cidan Huang, Jingkun Miao, Baosheng Zhu, Xiaohua Wang, Hui Zou, Lianshu Han, Jizhen Feng and Yonglan Huang
Int. J. Neonatal Screen. 2024, 10(2), 28; https://doi.org/10.3390/ijns10020028 - 29 Mar 2024
Abstract
►▼
Show Figures
The aim of this study was to observe the outcomes of newborn screening (NBS) in a certain population by using next-generation sequencing (NGS) as a first-tier screening test combined with tandem mass spectrometry (MS/MS). We performed a multicenter study of 29,601 newborns from
[...] Read more.
The aim of this study was to observe the outcomes of newborn screening (NBS) in a certain population by using next-generation sequencing (NGS) as a first-tier screening test combined with tandem mass spectrometry (MS/MS). We performed a multicenter study of 29,601 newborns from eight screening centers with NBS via NGS combined with MS/MS. A custom-designed panel targeting the coding region of the 142 genes of 128 inborn errors of metabolism (IEMs) was applied as a first-tier screening test, and expanded NBS using MS/MS was executed simultaneously. In total, 52 genes associated with the 38 IEMs screened by MS/MS were analyzed. The NBS performance of these two methods was analyzed and compared respectively. A total of 23 IEMs were diagnosed via NGS combined with MS/MS. The incidence of IEMs was approximately 1 in 1287. Within separate statistical analyses, the positive predictive value (PPV) for MS/MS was 5.29%, and the sensitivity was 91.3%. However, for genetic screening alone, the PPV for NGS was 70.83%, with 73.91% sensitivity. The three most common IEMs were methylmalonic academia (MMA), primary carnitine deficiency (PCD) and phenylketonuria (PKU). The five genes with the most common carrier frequencies were PAH (1:42), PRODH (1:51), MMACHC (1:52), SLC25A13 (1:55) and SLC22A5 (1:63). Our study showed that NBS combined with NGS and MS/MS improves the performance of screening methods, optimizes the process, and provides accurate diagnoses.
Full article
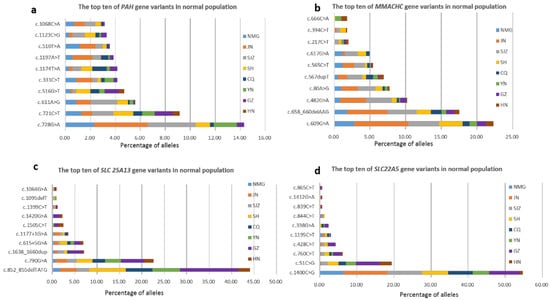
Figure 1
Open AccessArticle
Advancing Newborn Screening Long-Term Follow-Up: Integration of Epic-Based Registries, Dashboards, and Efficient Workflows
by
Katherine Raboin, Debra Ellis, Ginger Nichols, Marcia Hughes, Michael Brimacombe and Karen Rubin
Int. J. Neonatal Screen. 2024, 10(2), 27; https://doi.org/10.3390/ijns10020027 - 25 Mar 2024
Abstract
The Connecticut Newborn Screening (NBS) Network, in partnership with the Connecticut Department of Public Health, strategically utilized the Epic electronic health record (EHR) system to establish registries for tracking long-term follow-up (LTFU) of NBS patients. After launching the LTFU registry in 2019, the
[...] Read more.
The Connecticut Newborn Screening (NBS) Network, in partnership with the Connecticut Department of Public Health, strategically utilized the Epic electronic health record (EHR) system to establish registries for tracking long-term follow-up (LTFU) of NBS patients. After launching the LTFU registry in 2019, the Network obtained funding from the Health Resources and Services Administration to address the slow adoption by specialty care teams. An LTFU model was implemented in the three highest-volume specialty care teams at Connecticut Children’s, involving an early childhood cohort diagnosed with an NBS-identified disorder since the formation of the Network in March 2019. This cohort grew from 87 to 115 over the two-year project. Methods included optimizing registries, capturing external data from Health Information Exchanges, incorporating evidence-based guidelines, and conducting qualitative and quantitative evaluations. The early childhood cohort demonstrated significant and sustainable improvements in the percentage of visits up-to-date (%UTD) compared to the non-intervention legacy cohort of patients diagnosed with an NBS disorder before the formation of the Network. Positive trends in the early childhood cohort, including %UTD for visits and condition-specific performance metrics, were observed. The qualitative evaluation highlighted the achievability of practice behavior changes for specialty care teams through responsive support from the nurse analyst. The Network’s model serves as a use case for applying and achieving the adoption of population health tools within an EHR system to track care delivery and quickly fill identified care gaps, with the aim of improving long-term health for NBS patients.
Full article
(This article belongs to the Special Issue A Lifespan Approach to Health and Well-Being Leveraging Neonatal Screening: Efforts in Advocacy, Academia, Research, and Clinical Care)
►▼
Show Figures
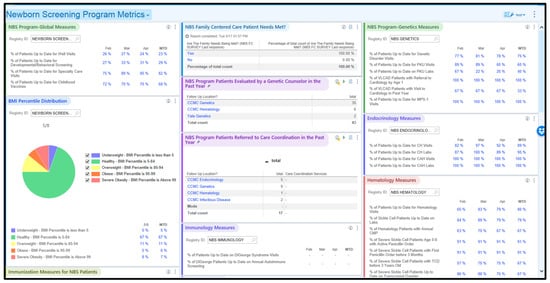
Figure 1
Open AccessArticle
Postponement of the Newborn Hearing Screening during the COVID-19 Pandemic; Parental Experiences and Worries
by
Rosanne B. van der Zee, Sanne L. Peet, Noëlle N. Uilenburg, Hedwig J. A. van Bakel and Evelien Dirks
Int. J. Neonatal Screen. 2024, 10(1), 26; https://doi.org/10.3390/ijns10010026 - 21 Mar 2024
Abstract
Early identification of hearing loss through newborn hearing screening followed by an early start of intervention has proven to be effective in promoting speech and language development in children with hearing loss. During the COVID-19 pandemic, newborn hearing screening was postponed for a
[...] Read more.
Early identification of hearing loss through newborn hearing screening followed by an early start of intervention has proven to be effective in promoting speech and language development in children with hearing loss. During the COVID-19 pandemic, newborn hearing screening was postponed for a group of newborns in the Netherlands. Therefore, meeting the guidelines for early identification was at risk. In this study, we examine parental attitudes, beliefs, and experiences concerning the hearing screening during the COVID-19 pandemic. Our results indicated that parents (n = 1053) were very positive about newborn hearing screening and their experiences with the screening, even during the COVID-19 pandemic. Parents’ beliefs on the information provision around newborn hearing screening were more inconsistent. The results showed that parents with a postponed hearing screening felt less informed about the hearing screening than parents without a postponed screening. Furthermore, child and family characteristics affected how parents experienced newborn hearing screening. Parents with a premature child were more worried about the hearing abilities of their child before the screening took place. The results also indicate that deafness in the family might lead to parental worries around newborn hearing screening.
Full article
Open AccessArticle
Portuguese Neonatal Screening Program: A Cohort Study of 18 Years Using MS/MS
by
Maria Miguel Gonçalves, Ana Marcão, Carmen Sousa, Célia Nogueira, Helena Fonseca, Hugo Rocha and Laura Vilarinho
Int. J. Neonatal Screen. 2024, 10(1), 25; https://doi.org/10.3390/ijns10010025 - 20 Mar 2024
Abstract
The Portuguese Neonatal Screening Program (PNSP) conducts nationwide screening for rare diseases, covering nearly 100% of neonates and screening for 28 disorders, including 24 inborn errors of metabolism (IEMs). The study’s purpose is to assess the epidemiology of the screened metabolic diseases and
[...] Read more.
The Portuguese Neonatal Screening Program (PNSP) conducts nationwide screening for rare diseases, covering nearly 100% of neonates and screening for 28 disorders, including 24 inborn errors of metabolism (IEMs). The study’s purpose is to assess the epidemiology of the screened metabolic diseases and to evaluate the impact of second-tier testing (2TT) within the PNSP. From 2004 to 2022, 1,764,830 neonates underwent screening using tandem mass spectrometry (MS/MS) to analyze amino acids and acylcarnitines in dried blood spot samples. 2TT was applied when necessary. Neonates with profiles indicating an IEM were reported to a reference treatment center, and subsequent biochemical and molecular studies were conducted for diagnostic confirmation. Among the screened neonates, 677 patients of IEM were identified, yielding an estimated birth prevalence of 1:2607 neonates. The introduction of 2TT significantly reduced false positives for various disorders, and 59 maternal cases were also detected. This study underscores the transformative role of MS/MS in neonatal screening, emphasizing the positive impact of 2TT in enhancing sensitivity, specificity, and positive predictive value. Our data highlight the efficiency and robustness of neonatal screening for IEM in Portugal, contributing to early and life-changing diagnoses.
Full article
(This article belongs to the Special Issue Neonatal Screening in Europe: On the Brink of a New Era)
►▼
Show Figures
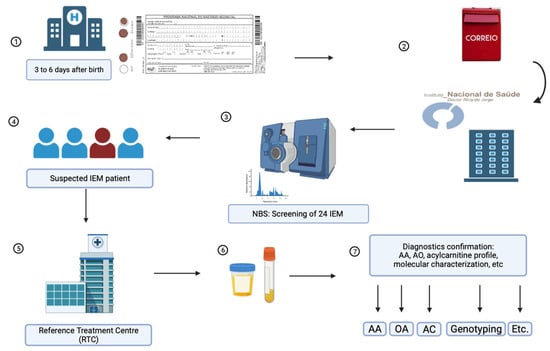
Figure 1
Open AccessArticle
Retrospective Review of Positive Newborn Screening Results for Isovaleric Acidemia and Development of a Strategy to Improve the Efficacy of Newborn Screening in the UK
by
Rachel S. Carling, Katy Hedgethorne, Anupam Chakrapani, Patricia L. Hall, Nick Flynn, Toby Greenfield, Stuart J. Moat, Joshua Ssali, Lynette Shakespeare, Nazia Taj, Teresa H. Y. Wu, Mark Anderson, Arunabha Ghosh, Hugh Lemonde, Germaine Pierre, Mark Sharrard, Sreevidya Sreekantam and James R. Bonham
Int. J. Neonatal Screen. 2024, 10(1), 24; https://doi.org/10.3390/ijns10010024 - 13 Mar 2024
Abstract
►▼
Show Figures
Since the UK commenced newborn screening for isovaleric acidemia in 2015, changes in prescribing have increased the incidence of false positive (FP) results due to pivaloylcarnitine. A review of screening results between 2015 and 2022 identified 24 true positive (TP) and 84 FP
[...] Read more.
Since the UK commenced newborn screening for isovaleric acidemia in 2015, changes in prescribing have increased the incidence of false positive (FP) results due to pivaloylcarnitine. A review of screening results between 2015 and 2022 identified 24 true positive (TP) and 84 FP cases, with pivalate interference confirmed in 76/84. Initial C5 carnitine (C5C) did not discriminate between FP and TP with median (range) C5C of 2.9 (2.0–9.6) and 4.0 (1.8–>70) µmol/L, respectively, and neither did Precision Newborn Screening via Collaborative Laboratory Integrated Reports (CLIR), which identified only 1/47 FP cases. However, among the TP cases, disease severity showed a correlation with initial C5C in ‘asymptomatic’ individuals (n = 17), demonstrating a median (range) C5C of 3.0 (1.8–7.1) whilst ‘clinically affected’ patients (n = 7), showed a median (range) C5C of 13.9 (7.7–70) µmol/L. These findings allowed the introduction of dual cut-off values into the screening algorithm to reduce the incidence of FPs, with initial C5C results ≥ 5 µmol/L triggering urgent referral, and those >2.0 and <5.0 µmol/L prompting second-tier C5-isobar testing. This will avoid delayed referral in babies at particular risk whilst reducing the FP rate for the remainder.
Full article
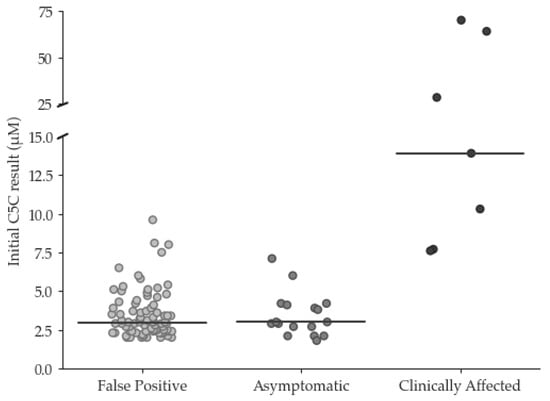
Figure 1
Open AccessArticle
Expanded Newborn Screening for Inborn Errors of Metabolism in Hong Kong: Results and Outcome of a 7 Year Journey
by
Kiran Moti Belaramani, Toby Chun Hei Chan, Edgar Wai Lok Hau, Matthew Chun Wing Yeung, Anne Mei Kwun Kwok, Ivan Fai Man Lo, Terry Hiu Fung Law, Helen Wu, Sheila Suet Na Wong, Shirley Wai Lam, Gladys Ha Yin Ha, Toby Pui Yee Lau, Tsz Ki Wong, Venus Wai Ching Or, Rosanna Ming Sum Wong, Wong Lap Ming, Jasmine Chi Kwan Chow, Eric Kin Cheong Yau, Antony Fu, Josephine Shuk Ching Chong, Ho Chung Yau, Grace Wing Kit Poon, Kwok Leung Ng, Kwong Tat Chan, Yuen Yu Lam, Joannie Hui, Chloe Miu Mak and Cheuk Wing Fungadd
Show full author list
remove
Hide full author list
Int. J. Neonatal Screen. 2024, 10(1), 23; https://doi.org/10.3390/ijns10010023 - 11 Mar 2024
Abstract
►▼
Show Figures
Newborn screening (NBS) is an important public health program that aims to identify pre-symptomatic healthy babies that will develop significant disease if left undiagnosed and untreated. The number of conditions being screened globally is expanding rapidly in parallel with advances in technology, diagnosis,
[...] Read more.
Newborn screening (NBS) is an important public health program that aims to identify pre-symptomatic healthy babies that will develop significant disease if left undiagnosed and untreated. The number of conditions being screened globally is expanding rapidly in parallel with advances in technology, diagnosis, and treatment availability for these conditions. In Hong Kong, NBS for inborn errors of metabolism (NBSIEM) began as a pilot program in October 2015 and was implemented to all birthing hospitals within the public healthcare system in phases, with completion in October 2020. The number of conditions screened for increased from 21 to 24 in April 2016 and then to 26 in October 2019. The overall recruitment rate of the NBS program was 99.5%. In the period between October 2015 and December 2022, 125,688 newborns were screened and 295 were referred back for abnormal results. The recall rate was reduced from 0.26% to 0.12% after the implementation of second-tier testing. An inherited metabolic disorder (IMD) was eventually confirmed in 47 infants, making the prevalence of IMD in Hong Kong 1 in 2674. At the time of the NBS result, 78.7% of the newborns with IMD were asymptomatic. There were two deaths reported: one newborn with methylmalonic acidemia cobalamin B type (MMACblB) died after the initial crisis and another case of carnitine palmitoyltransferase II deficiency (CPTII) died at 18 months of age after metabolic decompensation. The most common IMD noted were disorders of fatty acid oxidation metabolism (40%, 19 cases), closely followed by disorders of amino acid metabolism (38%, 18 cases), with carnitine uptake defect (19.1%, 9 cases) and citrullinemia type II (17%, 8 cases) being the two most common IMD picked up by the NBSIEM in Hong Kong. Out of the all the IMDs identified, 19.1% belonged to diverse ethnic groups. False negative cases were reported for citrullinemia type II and congenital adrenal hyperplasia during this period.
Full article
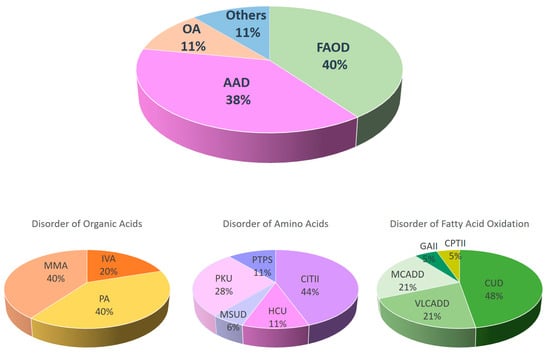
Figure 1
Open AccessArticle
Current Methods of Newborn Screening Follow-Up for Sickle Cell Disease Are Highly Variable and without Quality Assurance: Results from the ENHANCE Study
by
Najibah Galadanci, Shannon Phillips, Alyssa Schlenz, Nataliya Ivankova and Julie Kanter
Int. J. Neonatal Screen. 2024, 10(1), 22; https://doi.org/10.3390/ijns10010022 - 08 Mar 2024
Abstract
Newborn screening (NBS) for sickle cell disease (SCD) has significantly improved childhood survival but there are still gaps resulting in delayed care for affected infants. As a state-run program, there are no national quality assurance programs to ensure each state achieves consistent, reliable
[...] Read more.
Newborn screening (NBS) for sickle cell disease (SCD) has significantly improved childhood survival but there are still gaps resulting in delayed care for affected infants. As a state-run program, there are no national quality assurance programs to ensure each state achieves consistent, reliable outcomes. We performed this qualitative study of NBS follow-up practices to better evaluate and understand the multi-level, state-specific processes of how each state’s public health department delivers the NBS results to families, how/if they ensure affected infants are seen quickly by sickle cell specialists, and to determine the close-out processes used in each state. This project used semi-structured interviews conducted with 29 participants across eight states to explore these NBS follow-up processes in each state. Participants included SCD providers, NBS coordinators, or personnel associated with state health departments and community-based SCD organizations (CBO). Our results show significant state-dependent variations in the NBS processes of information delivery and patient management. Specifically, programs differed in how they communicated results to affected families and which other organizations were informed of the diagnosis. There was also state-based (and intrastate) variation in who should assume responsibility for ensuring that infants receive confirmatory testing and are promptly started on penicillin prophylaxis. Case closure was also highly variable and poorly validated. Our results also yielded identifiable challenges and facilitators to NBS which were highly variable by state but potentially addressable in the future. This information suggests opportunities for systematic improvement in NBS follow-up processes.
Full article
(This article belongs to the Special Issue A Lifespan Approach to Health and Well-Being Leveraging Neonatal Screening: Efforts in Advocacy, Academia, Research, and Clinical Care)
►▼
Show Figures
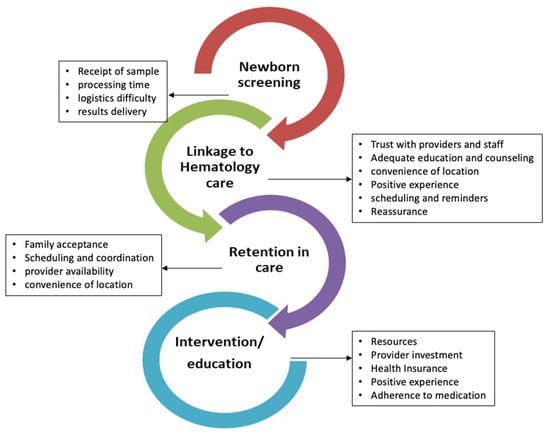
Figure 1
Open AccessArticle
Iowa Newborn Screening Program Experience with Hemoglobinopathy Screening over the Last Two Decades and Its Increasing Global Relevance
by
Ryan Jilek, Jennifer Marcy, Carol Johnson, Georgianne Younger, Amy Calhoun and Moon Ley Tung
Int. J. Neonatal Screen. 2024, 10(1), 21; https://doi.org/10.3390/ijns10010021 - 08 Mar 2024
Abstract
►▼
Show Figures
Hemoglobinopathies are the commonest monogenic disorder worldwide, with approximately seven percent of the world population being carriers of hemoglobinopathies. The healthcare utilization impact of thalassemia has resulted in global public health initiatives to screen for hemoglobinopathies, especially sickle cell disease (SCD). The Iowa
[...] Read more.
Hemoglobinopathies are the commonest monogenic disorder worldwide, with approximately seven percent of the world population being carriers of hemoglobinopathies. The healthcare utilization impact of thalassemia has resulted in global public health initiatives to screen for hemoglobinopathies, especially sickle cell disease (SCD). The Iowa Newborn Screening Program (INSP) has been in place for more than 50 years with a primary focus on detecting SCD. Recent changes in migration patterns have led to a global distribution of hemoglobinopathies in the western world, which has translated to an increase in the diagnosis of SCD and the incidental detection of non-sickling hemoglobinopathies within the INSP. This study documents the birth prevalence of hemoglobinopathies diagnosed in newborns through the INSP and highlights the need for newborn screening programs to evolve to meet the healthcare needs of underserved, minority populations.
Full article
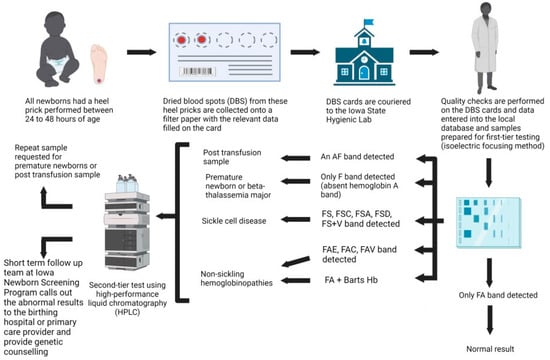
Figure 1
Open AccessArticle
Future of Dutch NGS-Based Newborn Screening: Exploring the Technical Possibilities and Assessment of a Variant Classification Strategy
by
Gea Kiewiet, Dineke Westra, Eddy N. de Boer, Emma van Berkel, Tom G. J. Hofste, Martine van Zweeden, Ronny C. Derks, Nico F. A. Leijsten, Martina H. A. Ruiterkamp-Versteeg, Bart Charbon, Lennart Johansson, Janneke Bos-Kruizinga, Inge J. Veenstra, Monique G. M. de Sain-van der Velden, Els Voorhoeve, M. Rebecca Heiner-Fokkema, Francjan van Spronsen, Birgit Sikkema-Raddatz and Marcel Nelen
Int. J. Neonatal Screen. 2024, 10(1), 20; https://doi.org/10.3390/ijns10010020 - 07 Mar 2024
Abstract
►▼
Show Figures
In this study, we compare next-generation sequencing (NGS) approaches (targeted panel (tNGS), whole exome sequencing (WES), and whole genome sequencing (WGS)) for application in newborn screening (NBS). DNA was extracted from dried blood spots (DBS) from 50 patients with genetically confirmed inherited metabolic
[...] Read more.
In this study, we compare next-generation sequencing (NGS) approaches (targeted panel (tNGS), whole exome sequencing (WES), and whole genome sequencing (WGS)) for application in newborn screening (NBS). DNA was extracted from dried blood spots (DBS) from 50 patients with genetically confirmed inherited metabolic disorders (IMDs) and 50 control samples. One hundred IMD-related genes were analyzed. Two data-filtering strategies were applied: one to detect only (likely) pathogenic ((L)P) variants, and one to detect (L)P variants in combination with variants of unknown significance (VUS). The variants were filtered and interpreted, defining true/false positives (TP/FP) and true/false negatives (TN/FN). The variant filtering strategies were assessed in a background cohort (BC) of 4833 individuals. Reliable results were obtained within 5 days. TP results (47 patient samples) for tNGS, WES, and WGS results were 33, 31, and 30, respectively, using the (L)P filtering, and 40, 40, and 38, respectively, when including VUS. FN results were 11, 13, and 14, respectively, excluding VUS, and 4, 4, and 6, when including VUS. The remaining FN were mainly samples with a homozygous VUS. All controls were TN. Three BC individuals showed a homozygous (L)P variant, all related to a variable, mild phenotype. The use of NGS-based workflows in NBS seems promising, although more knowledge of data handling, automated variant interpretation, and costs is needed before implementation.
Full article
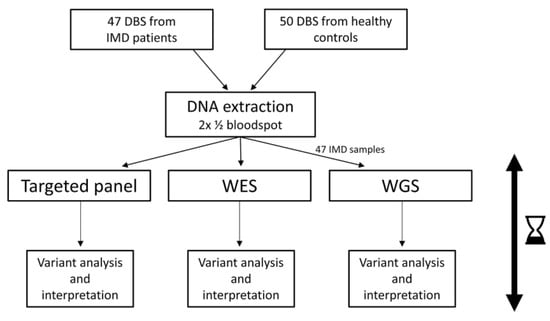
Figure 1
Open AccessArticle
Harnessing Next-Generation Sequencing as a Timely and Accurate Second-Tier Screening Test for Newborn Screening of Inborn Errors of Metabolism
by
Toby Chun Hei Chan, Chloe Miu Mak, Matthew Chun Wing Yeung, Eric Chun-Yiu Law, Jana Cheung, Tsz Ki Wong, Vincent Wing-Sang Cheng, Jacky Kwan Ho Lee, Jimmy Chi Lap Wong, Cheuk Wing Fung, Kiran Moti Belaramani, Anne Mei Kwun Kwok and Kwok Yeung Tsang
Int. J. Neonatal Screen. 2024, 10(1), 19; https://doi.org/10.3390/ijns10010019 - 05 Mar 2024
Abstract
►▼
Show Figures
In this study, we evaluated the implementation of a second-tier genetic screening test using an amplicon-based next-generation sequencing (NGS) panel in our laboratory during the period of 1 September 2021 to 31 August 2022 for the newborn screening (NBS) of six conditions for
[...] Read more.
In this study, we evaluated the implementation of a second-tier genetic screening test using an amplicon-based next-generation sequencing (NGS) panel in our laboratory during the period of 1 September 2021 to 31 August 2022 for the newborn screening (NBS) of six conditions for inborn errors of metabolism: citrullinemia type II (MIM #605814), systemic primary carnitine deficiency (MIM #212140), glutaric acidemia type I (MIM #231670), beta-ketothiolase deficiency (#203750), holocarboxylase synthetase deficiency (MIM #253270) and 3-hydroxy-3-methylglutaryl-CoA lyase deficiency (MIM # 246450). The custom-designed NGS panel can detect sequence variants in the relevant genes and also specifically screen for the presence of the hotspot variant IVS16ins3kb of SLC25A13 by the copy number variant calling algorithm. Genetic second-tier tests were performed for 1.8% of a total of 22,883 NBS samples. The false positive rate for these six conditions after the NGS second-tier test was only 0.017%, and two cases of citrullinemia type II would have been missed as false negatives if only biochemical first-tier testing was performed. The confirmed true positive cases were citrullinemia type II (n = 2) and systemic primary carnitine deficiency (n = 1). The false positives were later confirmed to be carrier of citrullinemia type II (n = 2), carrier of glutaric acidemia type I (n = 1) and carrier of systemic primary carnitine deficiency (n = 1). There were no false negatives reported. The incorporation of a second-tier genetic screening test by NGS greatly enhanced our program’s performance with 5-working days turn-around time maintained as before. In addition, early genetic information is available at the time of recall to facilitate better clinical management and genetic counseling.
Full article
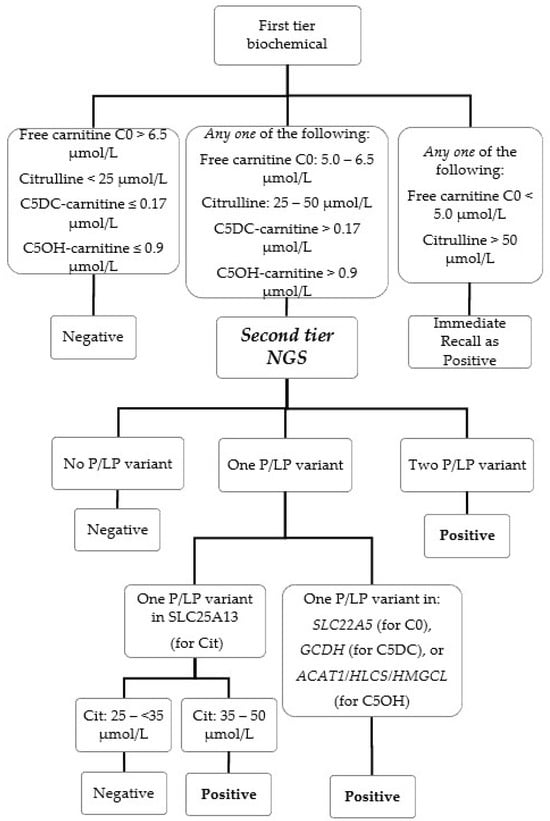
Figure 1
Open AccessArticle
Psychosocial Impact of a True-Positive, False-Positive, or Inconclusive Newborn Bloodspot Screening Result: A Questionnaire Study among Parents
by
Lieke M. van den Heuvel, Sylvia M. van der Pal, Rendelien K. Verschoof-Puite, Jasmijn E. Klapwijk, Ellen Elsinghorst, Eugènie Dekkers, Catharina P. B. van der Ploeg and Lidewij Henneman
Int. J. Neonatal Screen. 2024, 10(1), 18; https://doi.org/10.3390/ijns10010018 - 05 Mar 2024
Abstract
Expansion of newborn bloodspot screening (NBS) can increase health gain for more children but also increases the number of false-positive and uncertain results. The impact of abnormal and inconclusive NBS results on parental well-being and healthcare utilization was investigated. A questionnaire was sent
[...] Read more.
Expansion of newborn bloodspot screening (NBS) can increase health gain for more children but also increases the number of false-positive and uncertain results. The impact of abnormal and inconclusive NBS results on parental well-being and healthcare utilization was investigated. A questionnaire was sent to Dutch parents receiving an abnormal or inconclusive NBS result five weeks (T1) and four months (T2) post-NBS and compared to parents with a normal result (controls). In total, 35 true-positive (TP), 20 false-positive (FP), and 57 inconclusive (IC) participants and 268 controls filled out T1; 19 TP, 14 FP, 27 IC, and 116 controls filled out T2. Participants showed positive attitudes towards NBS. FP participants more often considered NBS less reliable. TP and FP participants experienced more negative emotions regarding the test result compared to controls at both T1 and T2, and IC only at T1. Parent-reported child vulnerability and perceptions of the newborn’s health status and of parenthood showed no differences. TP and FP participants reported more healthcare utilization at T1, and mainly TP at T2. TP and IC participants showed more emergency department visits at T1. The findings can be used to improve NBS programs and optimize support for families with various NBS results.
Full article
(This article belongs to the Special Issue Psychosocial Burden of Positive Newborn Screening)
►▼
Show Figures
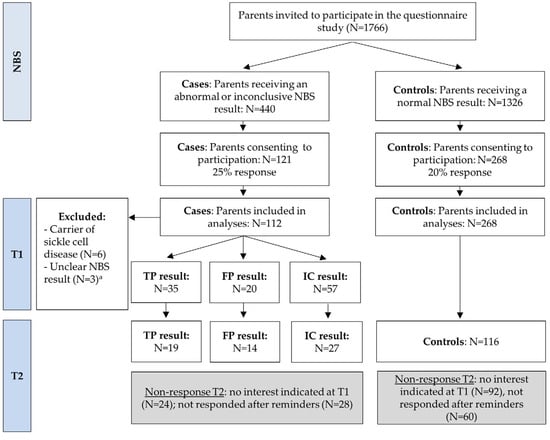
Figure 1
Open AccessArticle
New Cases of Maleylacetoacetate Isomerase Deficiency with Detection by Newborn Screening and Natural History over 32 Years: Experience from a German Newborn Screening Center
by
Gwendolyn Gramer, Saskia B. Wortmann, Junmin Fang-Hoffmann, Dirk Kohlmüller, Jürgen G. Okun, Holger Prokisch, Thomas Meitinger and Georg F. Hoffmann
Int. J. Neonatal Screen. 2024, 10(1), 17; https://doi.org/10.3390/ijns10010017 - 27 Feb 2024
Abstract
►▼
Show Figures
Newborn screening (NBS) for hepatorenal tyrosinemia type I (HT1) based on a determination of succinylacetone is performed in countries worldwide. Recently, biallelic pathogenic variants in GSTZ1 underlying maleylacetoacetate isomerase (MAAI) deficiency have been described as a differential diagnosis in individuals with slightly elevated
[...] Read more.
Newborn screening (NBS) for hepatorenal tyrosinemia type I (HT1) based on a determination of succinylacetone is performed in countries worldwide. Recently, biallelic pathogenic variants in GSTZ1 underlying maleylacetoacetate isomerase (MAAI) deficiency have been described as a differential diagnosis in individuals with slightly elevated succinylacetone detected by NBS. We report the experience with NBS for HT1 over 53 months in a large German NBS center and the identification and characterization of additional cases with MAAI deficiency, including one individual with a natural history over 32 years. A total of 516,803 children underwent NBS for HT1 at the NBS center in Heidelberg between August 2016 and December 2020. Of 42 children with elevated succinylacetone, HT1 was confirmed in two cases (1 in 258.401). MAAI deficiency was suspected in two cases and genetically confirmed in one who showed traces of succinylacetone in urine. A previously unreported pathogenic GSTZ1 variant was found in the index in a biallelic state. Segregation analysis revealed monoallelic carriership in the index case‘s mother and homozygosity in his father. The 32-year-old father had no medical concerns up to that point and the laboratory work-up was unremarkable. MAAI has to be considered a rare differential diagnosis in NBS for HT1 in cases with slight elevations of succinylacetone to allow for correct counselling and treatment decisions. Our observation of natural history over 32 years adds evidence for a benign clinical course of MAAI deficiency without specific treatment.
Full article
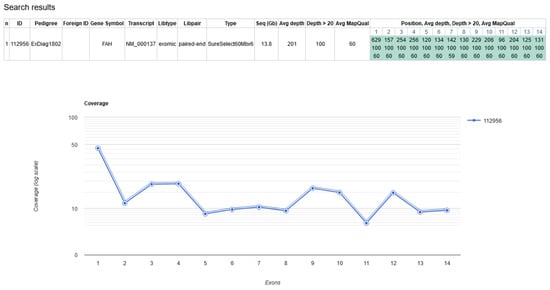
Figure 1
Open AccessArticle
History of Neonatal Screening of Congenital Hypothyroidism in Portugal
by
Maria José Costeira, Patrício Costa, Susana Roque, Ivone Carvalho, Laura Vilarinho and Joana Almeida Palha
Int. J. Neonatal Screen. 2024, 10(1), 16; https://doi.org/10.3390/ijns10010016 - 20 Feb 2024
Abstract
Congenital hypothyroidism (CH) leads to growth and development delays and is preventable with early treatment. Neonatal screening for CH was initiated in Portugal in 1981. This study examines the history of CH screening in the country. Data were obtained from annual reports and
[...] Read more.
Congenital hypothyroidism (CH) leads to growth and development delays and is preventable with early treatment. Neonatal screening for CH was initiated in Portugal in 1981. This study examines the history of CH screening in the country. Data were obtained from annual reports and from the national database of neonatal screening laboratory. The CH screening strategy primarily relies on the thyroid-stimulating hormone (TSH), followed by total thyroxine measurement as the second tier for confirmation. The TSH cutoff started at 90 mIU/L, decreasing to the actual 10 mIU/L. The coverage of the screening program has increased rapidly; although voluntary, it reached about 90% in 6 years and became universal in 10 years. Guideline and cutoff updates led to the identification of over 200 additional cases, resulting in specific retesting protocols for preterm and very-low-birth-weight babies. The actual decision tree considers CH when TSH levels are above 40 mIU/L. Data from the CH screening also provide an indication of the iodine status of the population, which is presently indicative of iodine insufficiency. The Portuguese neonatal screening for CH is a history of success. It has rapidly and continuously adapted to changes in knowledge and has become a universal voluntary practice within a few years.
Full article
(This article belongs to the Special Issue Neonatal Screening in Europe: On the Brink of a New Era)
►▼
Show Figures
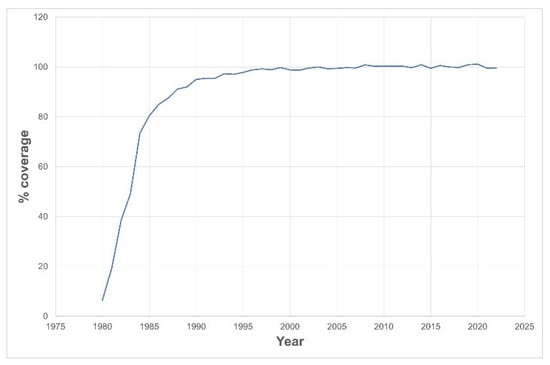
Figure 1
Open AccessArticle
Using the C14:1/Medium-Chain Acylcarnitine Ratio Instead of C14:1 to Reduce False-Positive Results for Very-Long-Chain Acyl-CoA Dehydrogenase Deficiency in Newborn Screening in Japan
by
Go Tajima, Junko Aisaki, Keiichi Hara, Miyuki Tsumura, Reiko Kagawa, Fumiaki Sakura, Hideo Sasai, Miori Yuasa, Yosuke Shigematsu and Satoshi Okada
Int. J. Neonatal Screen. 2024, 10(1), 15; https://doi.org/10.3390/ijns10010015 - 20 Feb 2024
Abstract
Very-long-chain acyl-CoA dehydrogenase (VLCAD) deficiency is a long-chain fatty acid oxidation disorder that manifests as either a severe phenotype associated with cardiomyopathy, a hypoglycemic phenotype, or a myopathic phenotype. As the hypoglycemic phenotype can cause sudden infant death, VLCAD deficiency is included in
[...] Read more.
Very-long-chain acyl-CoA dehydrogenase (VLCAD) deficiency is a long-chain fatty acid oxidation disorder that manifests as either a severe phenotype associated with cardiomyopathy, a hypoglycemic phenotype, or a myopathic phenotype. As the hypoglycemic phenotype can cause sudden infant death, VLCAD deficiency is included in newborn screening (NBS) panels in many countries. The tetradecenoylcarnitine (C14:1) level in dried blood specimens is commonly used as a primary marker for VLCAD deficiency in NBS panels. Its ratio to acetylcarnitine (C2) and various other acylcarnitines is used as secondary markers. In Japan, tandem mass spectrometry-based NBS, initially launched as a pilot study in 1997, was introduced to the nationwide NBS program in 2013. In the present study, we evaluated levels of acylcarnitine with various chain lengths (C18 to C2), free carnitine, and their ratios in 175 infants who tested positive for VLCAD deficiency with C14:1 and C14:1/C2 ratios. Our analyses indicated that the ratios of C14:1 to medium-chain acylcarnitines (C10, C8, and C6) were the most effective markers in reducing false-positive rates. Their use with appropriate cutoffs is expected to improve NBS performance for VLCAD deficiency.
Full article
(This article belongs to the Collection Newborn Screening in Japan)
►▼
Show Figures
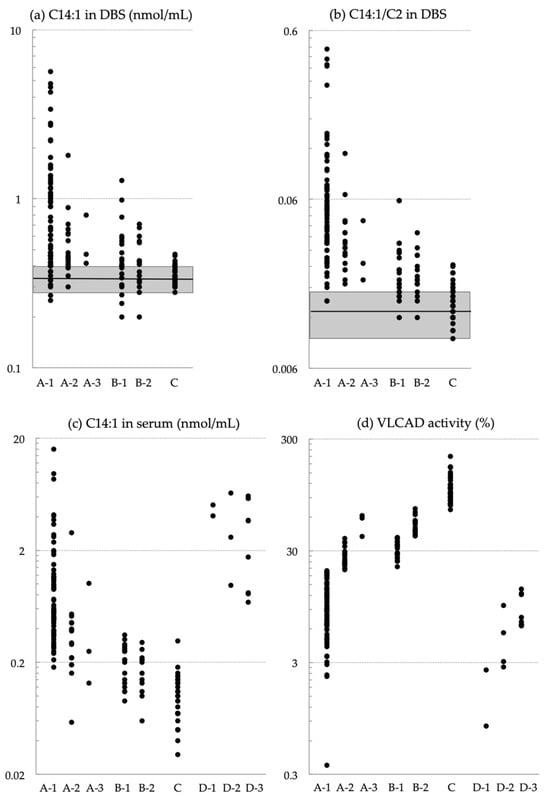
Figure 1
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics

Conferences
Special Issues
Special Issue in
IJNS
Newborn Screening for Disorders of Amino Acid Metabolism
Guest Editors: Raquel Yahyaoui, Cristiano RizzoDeadline: 31 May 2024
Special Issue in
IJNS
Newborn Screening for Congenital Hypothyroidism
Guest Editors: Ernest M. Post, Natasha Heather, Ralph FingerhutDeadline: 30 June 2024
Topical Collections
Topical Collection in
IJNS
Newborn Screening in Japan
Collection Editors: Toshihiro Tajima, Seiji Yamaguchi






