Asbestos Waste Treatment—An Effective Process to Selectively Recover Gold and Other Nonferrous Metals
Abstract
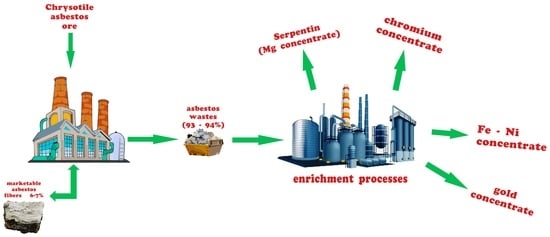
:1. Introduction
- Ensuring the profitability of the processing method, which is possible if the following three targets are met: the elimination of expensive “fine” crushing steps from the technological process, instead using only the simplest and cheapest methods of enrichment; the expansion of the range of produced marketable products; and the separation of the most expensive groups of noble and nonferrous metals.
- Realizing the least harmful (from an ecological point of view) method of processing possible by avoiding the use of active chemical reagents that are harmful to human health and the environment (acids, alkalis, etc.).
2. Experiment
2.1. Materials
2.2. Methods
Instrumental Analyses
3. Experimental Flowsheet
4. Results and Discussion
4.1. Results of Phase and Physico-Chemical Analysis of the Initial Tails
4.2. Results of Granulometric Analysis of the Initial Tails
- (1)
- Undersizing the heavy residue with a 0.25 mm sieve obtained a <0.25 mm yield of 30%, i.e., the partition process (separation) needed to be applied to 15 kg of tailings (see Figure 6). The analytical investigation showed that the concentration of gold in such a volume was 0.7 g/t (9.5 g), of which 50% was presumably in the form of gold nuggets; the rest comprised sulfides and magnetites.
- (2)
- The hydrocyclonic gravitational separation of the original heavy tailings using a 0.25 mm sieve isolated two products in approximately equal parts. The heavy residue with a mineral specific gravity of over 3.5 g/cm3 (collective gravity concentrate amounting to 50%) totaled about 7.5 kg. The float of roughly the same weight was sent to be leached for magnesium. Eighty percent or more of the heavy fraction comprised individualized minerals, which allowed for their subsequent separation into monomineralic products (concentrates) according to their physical properties.
- (3)
- The LIMS and HIMS of the collective heavy gravitational concentrate obtained two products: (a) 0.6 kg of magnetite concentrate for cast iron and steel smelting (with a yield amounting to 8% of the initial volume); and (b) a nonferrous fraction (92% of the initial volume) of about 6.9 kg, which should subsequently be subjected to LIMS. The magnetite content in the magnetite concentrate was 82–90%, and the iron content was 60–65%, which is sufficient for iron production.
- (4)
- The separation by LIMS of the 6.9 kg nonferrous fraction of the refuse, carried out via separators with a strong magnetic field, produced the following:
- A weak magnetic fraction, comprising a marketable chromite concentrate (0.138 kg, 10% of 1.38 kg) used to produce ferrochromium and chromite salts and an asphalt filler (0.138 kg, 10% of 1.38 kg).
- A nonmagnetic fraction of 5.52 kg (80% of the initial volume), comprising approximately 29% heavy silicates (olivine, forsterite, spinel, pyroxenes—1.59 kg); 51% light silicates (serpentine, 2.8 kg); 19.25% diopside (molding sands—1.06); small quantities (up to 1%) of nickel sulfides (millerite, pentlandite, pyrrhotite, etc.—0.06 kg); and gold (9.5 g). Chromite concentrate consists mainly of monomineral chromite with an insignificant admixture of pyrrhotite, magnetite, and serpentine. In the concentrate, the magnetite and serpentine are in the form of attachments.
- (5)
- The gravity concentration of the 5.52 kg of nonmagnetic fractions allowed the production of the following half-products: gold concentrate (0.153 kg) and silicate concentrate (5.51 kg). Both products should be sent for “honing”.
- Magnetite concentrate, suitable for iron production—0.6 kg;
- Marketable chromite concentrate—0.138 kg;
- Nickel–cobalt concentrate in the form of sulfides—0.01 kg;
- Diopside sand—1.06 kg;
- Olivine forsterite sand—1.59 kg;
- Gold concentrate—9.5 g.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baigenzhenov, O.; Kozlov, V.; Luganov, V.; Mishra, B.; Shayahmetova, R.; Aimbetova, I. Complex Processing of Tailings Generated in Chrysotile Asbestos Production. Min. Proc. Ext. Met. Rev. 2015, 36, 242–248. [Google Scholar] [CrossRef]
- Garside, M. Global Mine Production of Asbestos 2010–2021. 2022. Available online: https://www.statista.com/statistics/264924/world-mine-production-of-asbestos-since-2007/ (accessed on 12 October 2022).
- Fedoročková, A.; Raschman, P.; Sučik, G.; Ivánová, D.; Kavuličová, J. Utilization of Chrysotile-Type Tailings for Synthesis of High-Grade Silica by Controlled Precipitation. Min. Proc. Ext. Met. Rev. 2016, 37, 287–294. [Google Scholar] [CrossRef]
- Spasiano, D.; Pirozzi, F. Treatments of asbestos containing wastes. J. Environ. Manag. 2017, 204, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Foresti, E.; Fornero, E.; Lesci, I.; Rinaudo, C.; Zuccheri, T.; Roveri, N. Asbestos health hazard: A spectroscopic study of synthetic geo-inspired Fe-doped chrysotile. J. Hazard. Mater. 2009, 167, 1070–1079. [Google Scholar] [CrossRef]
- Jafarov, N.; Jafarov, F. Complex use of tailings deposits of chrysotile asbestos as a source for increasing the efficiency of production. Min. Geol. J. 2003, 3, 89–96. [Google Scholar]
- Obmiński, A. Asbestos waste recycling using the microwave technique—Benefits and risks. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100577. [Google Scholar] [CrossRef]
- Kjaerheim, K.; Ulvestad, B.; Martinsen, J.; Andersen, A. Cancer of the gastrointestinal tract and exposure to asbestos in drinking water among lighthouse keepers (Norway). Cancer Causes Control 2005, 16, 593–598. [Google Scholar] [CrossRef]
- Shevko, V.; Akylbekov, Y.; Karataeva, G.; Badikova, A. Recycling of chrysotile-asbestos production waste. Met. Res. Technol. 2022, 119, 410. [Google Scholar] [CrossRef]
- Kolesnikov, A.; Zhakipbaev, B.; Zhanikulov, N.; Kolesnikova, O.; Akhmetova, E.; Kuraev, R.; Shal, A. Review of technogenic waste and methods of its processing for the purpose of complex utilization of tailings from the enrichment of non-ferrous metal ores as a component of the raw material mixture in the production of cement clinker. Rasayan J. Chem. 2021, 14, 997–1005. [Google Scholar] [CrossRef]
- Lévesque, A.; Bélanger, N.; Poder, T.; Filotas, É.; Dupras, J. From white to green gold: Digging into public expectations and preferences for ecological restoration of asbestos mines in southeastern Quebec, Canada. Extr. Ind. Soc. 2020, 7, 1411–1423. [Google Scholar] [CrossRef]
- Barbosa, V.; Menezes, A.; Gedraite, R.; Ataíde, C. Vibration screening: A detailed study using image analysis techniques to characterize the bed behavior in solid–liquid separation. Min. Eng. 2020, 154, 106383. [Google Scholar] [CrossRef]
- Akizhayeva, A. Study of influence of the charge granulometric composition on the quality of burned anodes. Kompleks. Ispolz. Miner. Syra 2021, 319, 13–18. [Google Scholar] [CrossRef]
- Golmaei, M.; Kinnarinen, T.; Jernström, E.; Häkkinen, A. Efficient separation of hazardous trace metals and improvement of the filtration properties of green liquor dregs by a hydrocyclone. J. Clean. Prod. 2018, 183, 162–171. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, M.; Jiang, L.; Wang, H.; Xu, J.; Yang, J. High Concentration Fine Particle Separation Performance in Hydrocyclones. Minerals 2021, 11, 307. [Google Scholar] [CrossRef]
- Song, T.; Tian, J.; Ni, L.; Shen, C.; Yao, Y. Experimental study on liquid flow fields in de-foulant hydrocyclones with reflux ejector using particle image velocimetry. Sep. Purif. Technol. 2020, 240, 116555. [Google Scholar] [CrossRef]
- Pelevin, E.V. Technology of magnetite ore beneficiaiton and ways to improve the quality of iron concentrates. Min. J. 2011, 4, 20–28. [Google Scholar]
- Li, X.; Wang, Y.; Lu, D.; Zheng, X. Influence of Separation Angle on the Dry Pneumatic Magnetic Separation. Minerals 2022, 12, 1192. [Google Scholar] [CrossRef]
- Lu, D.; Liu, J.; Cheng, Z.; Li, X.; Xue, Z.; Li, S.; Zheng, X.; Wang, Y. Development of an Open-Gradient Magnetic Separator in the Aerodynamic Field. Physicochem. Probl. Miner. Process. 2020, 56, 13. [Google Scholar] [CrossRef]
- Xie, S.; Hu, Z.; Lu, D.; Zhao, Y. Dry Permanent Magnetic Separator: Present Status and Future Prospects. Minerals 2022, 12, 1251. [Google Scholar] [CrossRef]
- Ofori-Sarpong, G.; Amankwah, R. Comminution environment and gold particle morphology: Effects on gravity concentration. Miner. Eng. 2011, 24, 590–592. [Google Scholar] [CrossRef]
- Surimbayev, B.; Bolotova, L.; Shalgymbayev, S.; Razhan, E. Research of the complex stage-by-stage scheme of gravity separation of gold ore. News Natl. Acad. Sci. Repub. 2021, 5, 124–136. [Google Scholar] [CrossRef]
- Shalgymbayev, S.; Bolotova, L.; Surimbayev, B. Kazmekhanobr’s technologies in the field of processing of low-grade gold-bearing ores and technogenic raw materials. Tsvetnye Met. 2021, 38–45. [Google Scholar] [CrossRef]
- Toktar, G.; Koizhanova, A.; Magomedov, D.; Abdyldaev, N.; Bakraeva, A. Increased recovery of free fine gold in the leaching process. Kompleks. Ispolz. Miner. Syra 2022, 322, 51–58. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Fitzpatrick, R.; Kinchington, M.; Rollinson, G.; Hegarty, P. A Process Mineralogy Approach to Gravity Concentration of Tantalum Bearing Minerals. Minerals 2017, 7, 194. [Google Scholar] [CrossRef] [Green Version]






| Component | Formula | Weight, % |
|---|---|---|
| Serpentine | Mg3(Si2O5)(OH)4 | 53 |
| Brucite | Mg(OH)2 | 6 |
| Magnesium–nickel oxides | MgNiO2 | 2 |
| Antigorite | Mg6(Si4O10)(OH)8 | 23 |
| Magnesium oxide | MgO | 4 |
| Silimanite | Al2O3·SiO2 | 3 |
| Hematite | Fe2O3 | 5 |
| Vustite | FeO | 2 |
| Mg | Si | Al | Fe | Ca | Ni | Cr | Au, g/t |
|---|---|---|---|---|---|---|---|
| 23.4 | 17.8 | 0.42 | 5.3 | 0.34 | 0.19 | 0.15 | 0.3 |
| Fraction Size, mm | Class Output, % | Content of Components, % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MgO | SiO2 | Al2O3 | CaO | Fe3O4 | FeO | NiO | Cr2O3 | ||
| from 0.50 to 2.00 | 39 | 40.1 | 38.3 | 0.72 | 0.46 | 2.18 | 1.1 | 0.11 | 0.10 |
| from 0.25 to 0.50 | 33 | 39.7 | 38.9 | 0.82 | 0.49 | 2.87 | 1.6 | 0.23 | 0.14 |
| <0.25 | 28 | 39.0 | 36.1 | 0.85 | 0.44 | 5.2 | 2.1 | 0.25 | 0.23 |
| Name of the Product | Mass of the Product | |
|---|---|---|
| Obtained in the Laboratory Scale with a Volume of 100 kg of Chrysotile-Asbestos Tailings | Calculated for the Annual Yield of Chrysotile-Asbestos Tailings with a Volume of 3.2 Million Tons | |
| Magnetite concentrate | 0.6 kg | 19.2 thousand tons |
| Chromite concentrate | 0.138 kg | 4.41 thousand tons |
| Nickel–cobalt concentrate | 0.01 kg | 1.895 thousand tons |
| Diopside sand | 1.06 kg | 34 thousand tons |
| Olivine forsterite sand | 1.59 kg | 50.9 thousand tons |
| Gold concentrate | 9.5 g | 5 tons |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baigenzhenov, O.; Khabiyev, A.; Mishra, B.; Aimbetova, I.; Yulusov, S.; Temirgali, I.; Kuldeyev, Y.; Korganbayeva, Z. Asbestos Waste Treatment—An Effective Process to Selectively Recover Gold and Other Nonferrous Metals. Recycling 2022, 7, 85. https://doi.org/10.3390/recycling7060085
Baigenzhenov O, Khabiyev A, Mishra B, Aimbetova I, Yulusov S, Temirgali I, Kuldeyev Y, Korganbayeva Z. Asbestos Waste Treatment—An Effective Process to Selectively Recover Gold and Other Nonferrous Metals. Recycling. 2022; 7(6):85. https://doi.org/10.3390/recycling7060085
Chicago/Turabian StyleBaigenzhenov, Omirserik, Alibek Khabiyev, Brajendra Mishra, Indira Aimbetova, Sultan Yulusov, Inkar Temirgali, Yerzhan Kuldeyev, and Zhanar Korganbayeva. 2022. "Asbestos Waste Treatment—An Effective Process to Selectively Recover Gold and Other Nonferrous Metals" Recycling 7, no. 6: 85. https://doi.org/10.3390/recycling7060085