An Electrothermal Model of an NMC Lithium-Ion Prismatic Battery Cell for Temperature Distribution Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Thermal Model
2.2. The Electrical Model
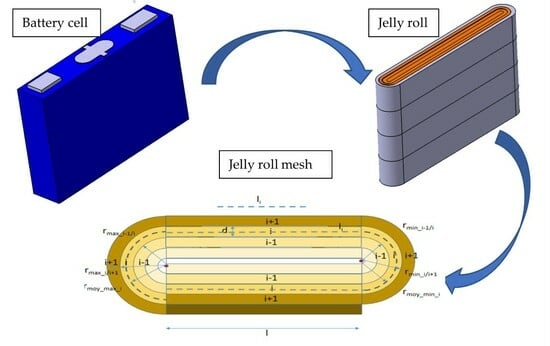
2.2.1. Electrical Model of a Meshed JR
2.2.2. Heat Generation Model
3. Results and Discussion
3.1. Validation of the JR Thermal Model
3.2. Validation of the Electrothermal Model of the Battery Cell
3.3. Thermal Gradient Assessment for a Given Current Profile
- The current profile is chosen to investigate the effect of current on the thermal gradient during charge and discharge;
- The battery is subjected to conduction through its bottom surface with a cooling plate;
- The air temperature is set at 25 °C;
- The cooling temperature is set at 30 °C.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- China Energy Storage Alliance. Energy Storage Industry White Paper. (Summary Version). 2022. Available online: https://en.cnesa.org/our-work (accessed on 26 March 2023).
- U.S. Geological Survey. Mineral Commodity Summaries 2023; Mineral Commodity Summaries No. 2023; U.S. Geological Survey: Reston, VA, USA, 2023. Available online: http://pubs.er.usgs.gov/publication/mcs2023 (accessed on 20 March 2023).
- Statista. Estimated Market Demand for Lithium-Ion Batteries Used in Electric Vehicles in 2019 with a Forecast for 2020 through 2030 (In Gigawatt Hours) [Graph]. 2021. Available online: https://www.statista.com/statistics/309570/lithium-ion-batterymarket-in-electric-vehicles/ (accessed on 13 March 2023).
- Scientific Background on the Nobel Prize in Chemistry. 2019. Available online: https://www.nobelprize.org/uploads/2019/10/advanced-chemistryprize2019-2.pdf (accessed on 9 October 2019).
- Statista. Estimated Electric Vehicle Battery Cell Price Breakdown as of 2020, by Category. 2020. Available online: https://www.statista.com/statistics/1176656/electric-vehicle-battery-price-by-category/?locale=en (accessed on 20 March 2023).
- Steinhardt, M.; Gillich, E.I.; Stiegler, M.; Jossen, A. Thermal conductivity inside prismatic lithium-ion cells with dependencies on temperature and external compression pressure. J. Energy Storage 2020, 32, 101680. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Xie, Z.; Wang, Y. Parametric Investigation on the Performance of a Battery Thermal Management System with Immersion Cooling. Energies 2022, 15, 2554. [Google Scholar] [CrossRef]
- Hunt, I.A.; Zhao, Y.; Patel, Y.; Offer, G.J. Surface Cooling Causes Accelerated Degradation Compared to Tab Cooling for Lithium-Ion Pouch Cells. J. Electrochem. Soc. 2016, 163, A1846–A1852. [Google Scholar] [CrossRef]
- Olabi, A.G.; Maghrabie, H.M.; Adhari, O.H.K.; Sayed, E.T.; Yousef, B.A.; Salameh, T.; Kamil, M.; Abdelkareem, M.A. Battery thermal management systems: Recent progress and challenges. Int. J. Thermofluids 2022, 15, 100171. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Z.; Qin, J.; Minqiang, P. A lightweight and low-cost liquid-cooled thermal management solution for high energy density prismatic lithium-ion battery packs. Appl. Therm. Eng. 2022, 203, 117871. [Google Scholar] [CrossRef]
- Wu, S.; Xiong, R.; Li, H.; Nian, V.; Ma, S. The state of the art on preheating lithium-ion batteries in cold weather. J. Energy Storage 2020, 27, 101059. [Google Scholar] [CrossRef]
- Kalogiannis, K.; Jaguemont, J.; Omar, N.; Mierlo, J.V.; Van den Bossche, P. A Comparison of Internal and External Preheat Methods for NMC Batteries. World Electr. Veh. J. 2019, 10, 18. [Google Scholar] [CrossRef]
- Shen, K.; Sun, J.; Zheng, Y.; Xu, C.; Wang, H.; Wang, S.; Chen, S.; Feng, X. A comprehensive analysis and experimental investigation for the thermal management of cell-to-pack battery system. Appl. Therm. Eng. 2022, 211, 118422. [Google Scholar] [CrossRef]
- Li, S.; Kirkaldy, N.; Zhang, C.; Gopalakrishnan, K.; Amietszajew, T.; Diaz, L.B.; Barreras, J.V.; Shams, M.; Hua, X.; Patel, Y.; et al. Optimal cell tab design and cooling strategy for cylindrical lithium-ion batteries. J. Power Sources 2021, 492, 229594. [Google Scholar] [CrossRef]
- Dao, T.-S.; Vyasarayani, C.P.; McPhee, J. Simplification and order reduction of lithium-ion battery model based on porous-electrode theory. J. Power Sources 2012, 198, 329–337. [Google Scholar] [CrossRef]
- Wang, D.; Huang, H.; Tang, Z.; Zhang, Q.; Yang, B.; Zhang, B. A lithium-ion battery electrochemical-thermal model for a wide temperature range applications. Electrochim. Acta 2020, 362, 137118. [Google Scholar] [CrossRef]
- Barreras, J.V.; Raj, T.; Howey, D.A. Derating strategies for lithium-ion batteries in electric vehicles. In Proceedings of the IECON 2018—44 th Annual Conference of the IEEE Industrial Electronics Society, Washington, DC, USA, 21–23 October 2018. [Google Scholar] [CrossRef]
- Perez Estevez, M.A.; Calligaro, S.; Bottesi, O.; Caligiuri, C. An electro-thermal model and its electrical parameters estimation procedure in a lithium-ion battery cell. J. Energy 2021, 234, 121296. [Google Scholar] [CrossRef]
- Shi, H.; Wang, S.; Fernandez, C.; Yu, C.; Xu, W.; Dablu, B.E.; Wang, L. Improved multi-time scale lumped thermoelectric coupling modeling and parameter dispersion evaluation of lithium-ion batteries. J. Appl. Energy 2022, 324, 119789. [Google Scholar] [CrossRef]
- Damay, N. Modélisation Thermique D’une Batterie Li-Ion Prismatique de Grande Capacité et Validation Expérimentale. Ph.D. Thesis, Université de Technologie de Compiègne, Compiègne, France, 2015; p. 8. [Google Scholar]
- Waldmann, T.; Scurtu, R.-G.; Richter, K.; Wohlfahrt-Mehrens, M. 18650 vs. 21700 Li-ion cells—A direct comparison of electrochemical, thermal, and geometrical properties. J. Power Sources 2020, 472, 228614. [Google Scholar] [CrossRef]
- Rezvanizaniani, S.M.; Liu, Z.; Chen, Y.; Lee, J. Review and recent advances in battery health monitoring and prognostics technologies for electric vehicle (EV) safety and mobility. J. Power Sources 2014, 256, 110–124. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Bohlen, O.; Baker, B. Aging Effect of Temperature Gradients in Li-ion Cells Experimental and Simulative Investigations and the Consequences on Thermal Battery Management. World Electr. Veh. J. 2012, 5, 322–333. [Google Scholar] [CrossRef]
- Worwood, D.; Kellner, Q.; Wojtala, M.; Widanage, W.D.; McGlen, R.; Greenwood, D.; Marco, J. A new approach to the internal thermal management of cylindrical battery cells for automotive applications. J. Power Sources 2017, 346, 151–166. [Google Scholar] [CrossRef]
- Madaoui, S.; Guillemard, F.; Sabatier, J.; Vinassa, J.-M. A detailed electro-thermal model of an NMC lithium-ion prismatic battery cell. In Proceedings of the 2023 IEEE 97th Vehicular Technology Conference (VTC2023-Spring), Florence, Italy, 20–23 June 2023. [Google Scholar] [CrossRef]
- Li, K.; Zhou, F.; Chen, X.; Yang, W.; Shen, J.; Song, Z. State-of-charge estimation combination algorithm for lithium-ion batteries with Frobenius-norm-based QR decomposition modified adaptive cubature Kalman filter and H-infinity filter based on electro-thermal model. J. Energy 2023, 263, 125763. [Google Scholar] [CrossRef]
- An, Z.; Zhang, C.; Luo, Y.; Zhang, J. Cooling and preheating behavior of compact power Lithium-ion battery thermal management system. J. Appl. Therm. Eng. 2023, 226, 120238. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y.; Shao, J.; Li, J.; Yu, Q. An electroechemical-thermal coupling model for prismatic lithium-ion batteries over wide temperature range. J. Appl. Therm. Eng. 2022, 217, 119282. [Google Scholar] [CrossRef]
- Zhao, Y.; Patel, Y.; Zhang, T.; Offer, G.J. Modeling the Effects of Thermal Gradients Induced by Tab and Surface Cooling on Lithium Ion Cell Performance. J. Electrochem. Soc. 2018, 165, A3169–A3178. [Google Scholar] [CrossRef]
- Skoog, S. Parameterization of equivalent circuit models for high power lithium-ion batteries in HEV applications. In Proceedings of the 2016 18th European Conference on Power Electronics and Applications (EPE’16 ECCE Europe), Karlsruhe, Germany, 5–9 September 2016; pp. 1–10. [Google Scholar] [CrossRef]
- Baccouche, I.; Jemmali, S.; Manai, B.; Nikolian, A.; Omar, N. Li-ion battery modeling and characterization: An experimental overview on NMC battery. Int. J. Energy Res. 2022, 46, 3843–3859. [Google Scholar] [CrossRef]
- Mathieu, R. Modélisation de L’influence de la Rapidité de Recharge Totale ou Partielle sur les Performances Electro-Thermiques et la Durée de vie des Batteries Pour Applications Automobiles. Ph.D. Thesis, Université de Bordeaux, Bordeaux, France, 2020; p. 253. [Google Scholar]
- Liu, G.; Ouyang, M.; Lu, L.; Li, J.; Han, X. Analysis of the heat generation of lithium-ion battery during charging and discharging considering different influencing factors. J. Therm. Anal. Calorim. 2014, 116, 1001–1010. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Palmieri, B.; Cilento, F.; Siviello, C.; Bertocchi, F.; Giordano, M.; Martone, A. Mitigation of Heat Propagation in a Battery Pack by Interstitial Graphite Nanoplatelet Layer: Coupled Electrochemical-Heat Transfer Model. J. Compos. Sci. 2022, 6, 296. [Google Scholar] [CrossRef]
- Wu, L.; Liu, K.; Liu, J.; Pang, H. Evaluating the heat generation characteristics of cylindrical lithium-ion battery considering the discharge rates and N/P ratio. J. Energy Storage 2023, 64, 107182. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.; Cao, L.; Yi, Z.; Li, P.; Guo, H. Heat generation and surrogate model for large-capacity nickel-rich prismatic lithium-ion battery as against 18650 battery. J. Loss Prev. Process Ind. 2022, 77, 104783. [Google Scholar] [CrossRef]
- Doh, C.-H.; Ha, Y.-C.; Eom, S.-W. Entropy measurement of a large format lithium ion battery and its application to calculate heat generation. Electrochim. Acta 2019, 309, 382–391. [Google Scholar] [CrossRef]
- Lv, Y.; Luo, W.; Li, C.; Zhang, G.; Huang, S.; Rao, Z. Experimental and numerical simulation study on the integrated thermal management system for electric vehicles. J. Energy Storage 2023, 70, 107895. [Google Scholar] [CrossRef]
- Zhao, J.; Lv, P.; Rao, Z. Experimental study on the thermal management performance of phase change material module for the large format prismatic lithium-ion battery. Energy 2022, 238, 122081. [Google Scholar] [CrossRef]
- Li, X.; Xiao, L.; Geng, G.; Jiang, Q. Temperature characterization based state-of-charge estimation for pouch lithium-ion battery. J. Power Sources 2022, 535, 231441. [Google Scholar] [CrossRef]
- Merveillaut, M. Modelisation non Entiere et non Lineaire d’un Accumulateur Litium-ion en vue de la Mise en œuvre D’observation de Variable Interne. Ph.D. Thesis, University of Bordeaux, Bordeaux, France, 2010. [Google Scholar]











| Specification | Value |
|---|---|
| Cathode material | NMC |
| Anode material | Graphite |
| Nominal capacity | 67 Ah |
| Cut-off voltages | 2.7–4.3 V |
| Dimensions | 150 mm 30 mm 100 mm |
| Thermal conductivities | 40–2.5–40 W·. |
| Thermal capacity | 1200 J··. |
| Material/Layer | Thickness [μm] | Density [kg·m−3] | Specific Heat Capacity [J·kg−1·K−1] | Thermal Conductivity [W·m−1·K−1] |
|---|---|---|---|---|
| Aluminum foil | 21 | 2702 | 903 | 238 |
| Copper foil | 12 | 8933 | 385 | 298 |
| Separator sheet | 25 | 1017 | 1978 | 0.34 |
| Positive electrode | 70 | 2895 | 1270 | 1.58 |
| Negative electrode | 79 | 1555 | 1437 | 1.04 |
| Instrument | Manufacturer | Type | Ultimate Range | Uncertainty |
|---|---|---|---|---|
| Battery cycler | BioLogic | BCS-815 | ±15 A/channel | ±1% |
| Thermocouples | RS PRO | K | −75~250 °C | ±1 °C |
| Climate chamber | Climats | TM 55 | −30~150 °C | ±0.15% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madaoui, S.; Vinassa, J.-M.; Sabatier, J.; Guillemard, F. An Electrothermal Model of an NMC Lithium-Ion Prismatic Battery Cell for Temperature Distribution Assessment. Batteries 2023, 9, 478. https://doi.org/10.3390/batteries9090478
Madaoui S, Vinassa J-M, Sabatier J, Guillemard F. An Electrothermal Model of an NMC Lithium-Ion Prismatic Battery Cell for Temperature Distribution Assessment. Batteries. 2023; 9(9):478. https://doi.org/10.3390/batteries9090478
Chicago/Turabian StyleMadaoui, Said, Jean-Michel Vinassa, Jocelyn Sabatier, and Franck Guillemard. 2023. "An Electrothermal Model of an NMC Lithium-Ion Prismatic Battery Cell for Temperature Distribution Assessment" Batteries 9, no. 9: 478. https://doi.org/10.3390/batteries9090478