Research Progress of Shear Thickening Electrolyte Based on Liquid–Solid Conversion Mechanism
Abstract
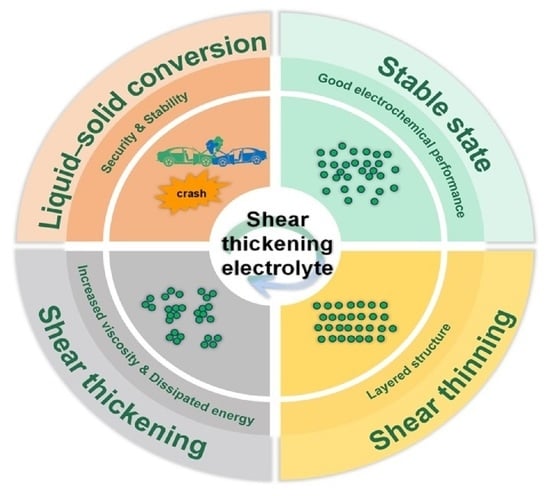
:1. Introduction
2. Shear Thickening Mechanism
3. Shear Thickening Electrolyte
4. Conclusions
5. Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalhoff, J.; Eshetu, G.G.; Bresser, D.; Passerini, S. Safer Electrolytes for Lithium-Ion Batteries: State of the Art and Perspectives. ChemSusChem 2015, 8, 2154–2175. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, P.G.; Ramesh, R.; Prem Kumar, T. Safety Mechanisms in Lithium-Ion Batteries. J. Power Sources 2006, 155, 401–414. [Google Scholar] [CrossRef]
- Ming, J.; Cao, Z.; Wu, Y.; Wahyudi, W.; Wang, W.; Guo, X.; Cavallo, L.; Hwang, J.-Y.; Shamim, A.; Li, L.-J.; et al. New Insight on the Role of Electrolyte Additives in Rechargeable Lithium Ion Batteries. ACS Energy Lett. 2019, 4, 2613–2622. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for Lithium-Ion Battery Safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, D.; Chen, M.; Huang, Q.; Weng, J.; Wang, Z.; Wang, J. A Review on the Thermal Hazards of the Lithium-Ion Battery and the Corresponding Countermeasures. Appl. Sci. 2019, 9, 2483. [Google Scholar] [CrossRef] [Green Version]
- Arora, S.; Shen, W.; Kapoor, A. Review of Mechanical Design and Strategic Placement Technique of a Robust Battery Pack for Electric Vehicles. Renew. Sustain. Energy Rev. 2016, 60, 1319–1331. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Fan, H.; Wang, H. Flame-Retardant Electrolyte Solution for Dual-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 1363–1370. [Google Scholar] [CrossRef]
- Deng, K.; Zeng, Q.; Wang, D.; Liu, Z.; Wang, G.; Qiu, Z.; Zhang, Y.; Xiao, M.; Meng, Y. Nonflammable Organic Electrolytes for High-Safety Lithium-Ion Batteries. Energy Storage Mater. 2020, 32, 425–447. [Google Scholar] [CrossRef]
- Hyung, Y.E.; Vissers, D.R.; Amine, K. Flame-Retardant Additives for Lithium-Ion Batteries. J. Power Sources 2003, 119, 383–387. [Google Scholar] [CrossRef]
- Xiang, H.; Jin, Q.; Chen, C.H.; Ge, X.; Guo, S.; Sun, J. Dimethyl Methylphosphonate-Based Nonflammable Electrolyte and High Safety Lithium-Ion Batteries. J. Power Sources 2007, 174, 335–341. [Google Scholar] [CrossRef]
- Kizilel, R.; Sabbah, R.; Selman, J.R.; Al-Hallaj, S. An Alternative Cooling System to Enhance the Safety of Li-Ion Battery Packs. J. Power Sources 2009, 194, 1105–1112. [Google Scholar] [CrossRef]
- Kizilel, R.; Lateef, A.; Sabbah, R.; Farid, M.; Selman, J.; Al-Hallaj, S. Passive Control of Temperature Excursion and Uniformity in High-Energy Li-Ion Battery Packs at High Current and Ambient Temperature. J. Power Sources 2008, 183, 370–375. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Forsyth, M.; Howlett, P.C.; Kar, M.; Passerini, S.; Pringle, J.M.; Ohno, H.; Watanabe, M.; Yan, F.; Zheng, W.; et al. Ionic Liquids and Their Solid-State Analogues as Materials for Energy Generation and Storage. Nat. Rev. Mater. 2016, 1, 15005. [Google Scholar] [CrossRef]
- Shu, K.; Wang, C.; Li, W.; Bussell, T.; Ding, J. Electrolytes with Reversible Switch between Liquid and Solid Phases. Curr. Opin. Electrochem. 2020, 21, 297–302. [Google Scholar] [CrossRef]
- Veith, G.M.; Armstrong, B.L.; Wang, H.; Kalnaus, S.; Tenhaeff, W.E.; Patterson, M.L. Shear Thickening Electrolytes for High Impact Resistant Batteries. ACS Energy Lett. 2017, 2, 2084–2088. [Google Scholar] [CrossRef]
- Zhang, S.S. A Review on Electrolyte Additives for Lithium-Ion Batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar] [CrossRef]
- Haregewoin, A.M.; Wotango, A.S.; Hwang, B.-J. Electrolyte Additives for Lithium Ion Battery Electrodes: Progress and Perspectives. Energy Environ. Sci. 2016, 9, 1955–1988. [Google Scholar] [CrossRef]
- Barnes, H. Shear-Thickening (“Dilatancy”) in Suspensions of Nonaggregating Solid Particles Dispersed in Newtonian Liquids. J. Rheol. 1989, 33, 329–366. [Google Scholar] [CrossRef]
- Wyart, M.; Cates, M.E. Discontinuous Shear Thickening without Inertia in Dense Non-Brownian Suspensions. Phys. Rev. Lett. 2014, 112, 098302. [Google Scholar] [CrossRef] [Green Version]
- Bag, N.; Bhattacharyya, S. Electroosmotic Flow of a Non-Newtonian Fluid in a Microchannel with Heterogeneous Surface Potential. J. Non-Newton. Fluid Mech. 2018, 259, 48–60. [Google Scholar] [CrossRef]
- Wagner, N.J.; Brady, J.F. Shear Thickening in Colloidal Dispersions. Phys. Today 2009, 62, 27–32. [Google Scholar] [CrossRef]
- Olsson, P.; Teitel, S. Critical Scaling of Shear Viscosity at the Jamming Transition. Phys. Rev. Lett. 2007, 99, 178001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Cheng, C.-F.; Zhou, L.; Zou, F.; Liang, W.; Wang, M.; Zhu, Y. A Shear Thickening Fluid Based Impact Resistant Electrolyte for Safe Li-Ion Batteries. J. Power Sources 2019, 423, 297–304. [Google Scholar] [CrossRef]
- Ye, Y.; Xiao, H.; Reaves, K.; McCulloch, B.; Mike, J.F.; Lutkenhaus, J.L. Effect of Nanorod Aspect Ratio on Shear Thickening Electrolytes for Safety-Enhanced Batteries. ACS Appl. Nano Mater. 2018, 1, 2774–2784. [Google Scholar] [CrossRef]
- Raghavan, S.R.; Walls, H.J.; Khan, S.A. Rheology of Silica Dispersions in Organic Liquids: New Evidence for Solvation Forces Dictated by Hydrogen Bonding. Langmuir 2000, 16, 7920–7930. [Google Scholar] [CrossRef]
- Li, X.; Cao, H.; Gao, S.; Pan, F.; Weng, L.; Song, S.; Huang, Y. Preparation of Body Armour Material of Kevlar Fabric Treated with Colloidal Silica Nanocomposite. Plast. Rubber Compos. 2008, 37, 223–226. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Gong, X. The Rheology of Shear Thickening Fluid (Stf) and the Dynamic Performance of an Stf-Filled Damper. Smart Mater. Struct. 2008, 17, 035027. [Google Scholar] [CrossRef]
- Gürgen, S.; Kuşhan, M.C.; Li, W. Shear Thickening Fluids in Protective Applications: A Review. Prog. Polym. Sci. 2017, 75, 48–72. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, Y.; Zhou, J.; Wu, J.; Liu, S.; Sang, M.; Liu, B.; Pan, Y.; Gong, X. Multi-Functional Stf-Based Yarn for Human Protection and Wearable Systems. Chem. Eng. J. 2023, 453, 139869. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Q.; Pan, Y.; Zhou, J.; Zhang, J.; Liu, S.; Fan, Z.; Deng, H.; Hu, Y.; Gong, X. An Impact-Resistant and Flame-Retardant Cnts/Stf/Kevlar Composite with Conductive Property for Safe Wearable Design. Compos. Part A Appl. Sci. Manuf. 2023, 168, 107489. [Google Scholar] [CrossRef]
- Zheng, Y.; Yin, R.; Zhao, Y.; Liu, H.; Zhang, D.; Shi, X.; Zhang, B.; Liu, C.; Shen, C. Conductive Mxene/Cotton Fabric Based Pressure Sensor with Both High Sensitivity and Wide Sensing Range for Human Motion Detection and E-Skin. Chem. Eng. J. 2021, 420, 127720. [Google Scholar] [CrossRef]
- Zarei, M.; Aalaie, J. Application of Shear Thickening Fluids in Material Development. J. Mater. Res. Technol. 2020, 9, 10411–10433. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Gong, X. Study on Magnetorheological Shear Thickening Fluid. Smart Mater. Struct. 2008, 17, 015051. [Google Scholar] [CrossRef] [Green Version]
- Williams, T.H.; Day, J.; Pickard, S. Surgical and Medical Garments and Materials Incorporating Shear Thickening Fluids. U.S. Patent 12/440,086, 27 April 2009. [Google Scholar]
- Liu, B.; Shelley, M.; Zhang, J. Focused Force Transmission through an Aqueous Suspension of Granules. Phys. Rev. Lett. 2010, 105, 188301. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Sang, M.; Shu, Q.; Zhang, J.; Xuan, S.; Gong, X. A Safeguarding and High Temperature Tolerant Organogel Electrolyte for Flexible Solid-State Supercapacitors. J. Power Sources 2021, 505, 230083. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, J.; Sheng, W.; Wang, F.; Zhang, J.; Zhu, F.; Zhuang, X.; Jordan, R.; Schmidt, O.G.; Feng, X. Thermoswitchable on-Chip Microsupercapacitors: One Potential Self-Protection Solution for Electronic Devices. Energy Environ. Sci. 2018, 11, 1717–1722. [Google Scholar] [CrossRef]
- Zhu, M.; Meng, W.; Huang, Y.; Zhi, C. Proton-Insertion-Enhanced Pseudocapacitance Based on the Assembly Structure of Tungsten Oxide. ACS Appl. Mater. Interfaces 2014, 6, 18901–18910. [Google Scholar] [CrossRef]
- Wang, X.; Wang, P.; Jiang, Y.; Su, Q.; Zheng, J. Facile Surface Modification of Silica Nanoparticles with a Combination of Noncovalent and Covalent Methods for Composites Application. Compos. Sci. Technol. 2014, 104, 1–8. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Su, Q.; Zheng, J. Use of Unmodified Sio2 as Nanofiller to Improve Mechanical Properties of Polymer-Based Nanocomposites. Compos. Sci. Technol. 2013, 89, 52–60. [Google Scholar] [CrossRef]
- Tripathi, A.M.; Su, W.-N.; Hwang, B.J. In Situ Analytical Techniques for Battery Interface Analysis. Chem. Soc. Rev. 2018, 47, 736–851. [Google Scholar] [CrossRef]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of Inorganic Solid-State Electrolytes for Batteries. Nat. Mater. 2019, 18, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Isaac, J.A.; Devaux, D.; Bouchet, R. Dense Inorganic Electrolyte Particles as a Lever to Promote Composite Electrolyte Conductivity. Nat. Mater. 2022, 21, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Y.; Yi, Q.; Wang, X.; Camacho, R.A.P.; Kungl, H.; Eichel, R.A.; Lu, L.; Zhang, H. Ion Conduction in Composite Polymer Electrolytes: Potential Electrolytes for Sodium-Ion Batteries. ChemSusChem 2023, 16, e202202152. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, J.; Tang, Z.; Wang, H.; Lian, H.; Zhang, J.; Cao, C.-n. Physicochemical Properties of Poly (Ethylene Oxide)-Based Composite Polymer Electrolytes with a Silane-Modified Mesoporous Silica Sba-15. Electrochim. Acta 2009, 54, 3490–3494. [Google Scholar] [CrossRef]
- Chen-Yang, Y.; Wang, Y.; Chen, Y.; Li, Y.; Chen, H.; Chiu, H. Influence of Silica Aerogel on the Properties of Polyethylene Oxide-Based Nanocomposite Polymer Electrolytes for Lithium Battery. J. Power Sources 2008, 182, 340–348. [Google Scholar] [CrossRef]
- Croce, F.; Appetecchi, G.; Persi, L.; Scrosati, B. Nanocomposite Polymer Electrolytes for Lithium Batteries. Nature 1998, 394, 456–458. [Google Scholar] [CrossRef]
- Jayanthi, S.; Shenbagavalli, S.; Muthuvinayagam, M.; Sundaresan, B. Effect of Nano Tio2 on the Thransport, Structural and Thermal Properties of Pema-Nai Solid Polymer Electrolytes for Energy Storage Devices. Mater. Sci. Eng. B 2022, 285, 115942. [Google Scholar] [CrossRef]
- Volfkovich, Y.M.; Rychagov, A.Y.; Sosenkin, V.E.; Baskakov, S.A.; Kabachkov, E.N.; Shulga, Y.M. Supercapacitor Properties of Rgo-Tio2 Nanocomposite in Two-Component Acidic Electrolyte. Materials 2022, 15, 7856. [Google Scholar] [CrossRef]
- Bronstein, L.M.; Karlinsey, R.L.; Ritter, K.; Joo, C.G.; Stein, B.; Zwanziger, J.W. Design of Organic–Inorganic Solid Polymer Electrolytes: Synthesis, Structure, and Properties. J. Mater. Chem. 2004, 14, 1812–1820. [Google Scholar] [CrossRef]
- Kumar, B.; Fellner, J. Polymer–Ceramic Composite Protonic Conductors. J. Power Sources 2003, 123, 132–136. [Google Scholar] [CrossRef]
- Charradi, K.; Ahmed, Z.; Aranda, P.; Chtourou, R. Silica/Montmorillonite Nanoarchitectures and Layered Double Hydroxide-Speek Based Composite Membranes for Fuel Cells Applications. Appl. Clay Sci. 2019, 174, 77–85. [Google Scholar] [CrossRef]
- Ding, J.; Tian, T.; Meng, Q.; Guo, Z.; Li, W.; Zhang, P.; Ciacchi, F.T.; Huang, J.; Yang, W. Smart Multifunctional Fluids for Lithium Ion Batteries: Enhanced Rate Performance and Intrinsic Mechanical Protection. Sci. Rep. 2013, 3, 2485. [Google Scholar] [CrossRef] [Green Version]
- Xiaoxia, C.; Kai, L.; Baoguo, W. Research on High-Safety Electrolytes and Their Application in Lithium-Ion Batteries. Energy Storage Sci. Technol. 2020, 9, 583–592. [Google Scholar] [CrossRef]
- Yu, O.; Wenhui, H.; Kai, L. Research Progress of Smart Safety Electrolytes in Lithium-Ion Batteries. Energy Storage Sci. Technol. 2022, 11, 1772. [Google Scholar] [CrossRef]
- Heo, Y.; Larson, R.G. The Scaling of Zero-Shear Viscosities of Semidilute Polymer Solutions with Concentration. J. Rheol. 2005, 49, 1117–1128. [Google Scholar] [CrossRef]
- Srivastava, A.; Majumdar, A.; Butola, B.S. Improving the Impact Resistance of Textile Structures by Using Shear Thickening Fluids: A Review. Crit. Rev. Solid State Mater. Sci. 2012, 37, 115–129. [Google Scholar] [CrossRef]
- Dong, H.; Hu, X.; He, G. A Shear-Thickening Colloidal Electrolyte for Aqueous Zinc-Ion Batteries with Resistance on Impact. Nanoscale 2022, 14, 14544–14551. [Google Scholar] [CrossRef]
- Hamaker, H.C. The London—Van Der Waals Attraction between Spherical Particles. Physica 1937, 4, 1058–1072. [Google Scholar] [CrossRef]
- Mewis, J.; Biebaut, G. Shear Thickening in Steady and Superposition Flows Effect of Particle Interaction Forces. J. Rheol. 2001, 45, 799–813. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; Liang, S.; Lu, B.; Zhou, J. Ph-Buffer Contained Electrolyte for Self-Adjusted Cathode-Free Zn–Mno2 Batteries with Coexistence of Dual Mechanisms. Small Struct. 2021, 2, 2100119. [Google Scholar] [CrossRef]
- Yufit, V.; Tariq, F.; Eastwood, D.S.; Biton, M.; Wu, B.; Lee, P.D.; Brandon, N.P. Operando Visualization and Multi-Scale Tomography Studies of Dendrite Formation and Dissolution in Zinc Batteries. Joule 2019, 3, 485–502. [Google Scholar] [CrossRef] [Green Version]
- Hsu, P.-C.; Seol, S.-K.; Lo, T.-N.; Liu, C.-J.; Wang, C.-L.; Lin, C.-S.; Hwu, Y.; Chen, C.; Chang, L.-W.; Je, J.; et al. Hydrogen Bubbles and the Growth Morphology of Ramified Zinc by Electrodeposition. J. Electrochem. Soc. 2008, 155, D400. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.; Guo, S.; He, P.; Zhou, H. Status and Prospects of Polymer Electrolytes for Solid-State Li–O2 (Air) Batteries. Energy Environ. Sci. 2017, 10, 860–884. [Google Scholar] [CrossRef]
- Zheng, G.; Yan, T.; Hong, Y.; Zhang, X.; Wu, J.; Liang, Z.; Cui, Z.; Du, L.; Song, H. A Non-Newtonian Fluid Quasi-Solid Electrolyte Designed for Long Life and High Safety Li-O(2) Batteries. Nat. Commun. 2023, 14, 2268. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Liang, X.; Liu, B.; Deng, H. Research Progress of Shear Thickening Electrolyte Based on Liquid–Solid Conversion Mechanism. Batteries 2023, 9, 384. https://doi.org/10.3390/batteries9070384
Huang Q, Liang X, Liu B, Deng H. Research Progress of Shear Thickening Electrolyte Based on Liquid–Solid Conversion Mechanism. Batteries. 2023; 9(7):384. https://doi.org/10.3390/batteries9070384
Chicago/Turabian StyleHuang, Qianqian, Xin Liang, Bing Liu, and Huaxia Deng. 2023. "Research Progress of Shear Thickening Electrolyte Based on Liquid–Solid Conversion Mechanism" Batteries 9, no. 7: 384. https://doi.org/10.3390/batteries9070384