Experimental Investigation on the Thermal Management for Lithium-Ion Batteries Based on the Novel Flame Retardant Composite Phase Change Materials
Abstract
:1. Introduction
2. Materials Preparation and Experiment
2.1. Materials
2.2. Preparation of the Hybrids Containing PA, HDPE, EG, and FR
2.3. Characterization of the Flame Retardant CPCMs
2.3.1. Chemical Characterization and Thermophysical Properties
2.3.2. Flame Retardant Properties
2.4. Experimental Setup
3. Results and Discussions
3.1. The Morphology, Chemical Characterization, and Thermal Properties
3.2. The Flammability Test of CPCMs
3.3. Thermal Management Characteristics
3.3.1. Cell
3.3.2. Module
4. Conclusions
- (1)
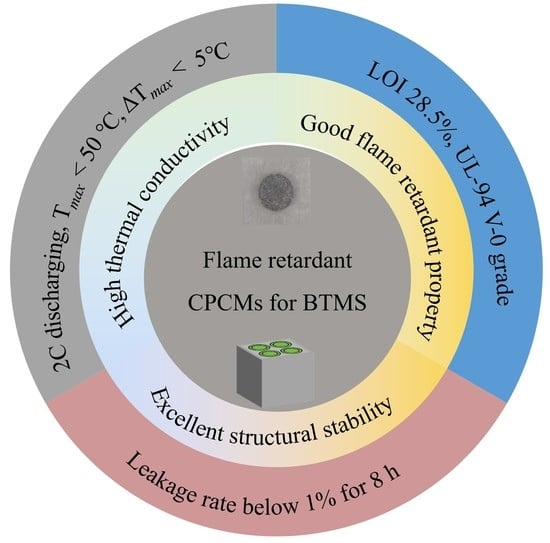
- XRD results show that the mixture between the PCM and the additives in the flame retardant CPCM made in this experiment is a physical mixture, and no chemical reaction occurs. The flame retardant CPCM has excellent structural stability, and after eight hours of continuous heat, the leakage rate is kept within 1%.
- (2)
- CPCM25 with 25 wt% flame retardant achieved a V-0 rating in the UL-94 test, a thermal conductivity of 0.77 W·m−1·K−1, and a latent heat value of 87.63 J/g. In the thermogravimetric test, the residue at 800 °C reached 25.22%.
- (3)
- Battery modules with flame retardant CPCMs offer excellent thermal management. Under a 2C discharge rate, the Tmax of the battery pack remains below 50 °C, and the ΔTmax can be controlled within 5 °C. Even under a 3C discharge rate, the Tmax and ΔTmax are reduced by 30.31% and 29.53%, respectively.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Caineng, Z.O.; Xiong, B.; Huaqing, X.U.; Zheng, D.; Zhixin, G.E.; Ying, W.A.; Jiang, L.; Songqi, P.; Songtao, W. The role of new energy in carbon neutral. Pet. Explor. Dev. 2021, 48, 411–420. [Google Scholar]
- Wei, Y.M.; Chen, K.; Kang, J.N.; Chen, W.; Wang, X.Y.; Zhang, X. Policy and Management of Carbon Peaking and Carbon Neutrality: A Literature Review. Engineering 2022, 14, 52–63. [Google Scholar] [CrossRef]
- Holechek, J.L.; Geli, H.M.E.; Sawalhah, M.N.; Valdez, R. A Global Assessment: Can Renewable Energy Replace Fossil Fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- Chen, J.; Su, F.; Jain, V.; Salman, A.; Tabash, M.I.; Haddad, A.M.; Zabalawi, E.; Abdalla, A.A.; Shabbir, M.S. Does Renewable Energy Matter to Achieve Sustainable Development Goals? The Impact of Renewable Energy Strategies on Sustainable Economic Growth. Front. Energy Res. 2022, 10, 829252. [Google Scholar] [CrossRef]
- Liu, W.; Placke, T.; Chau, K.T. Overview of batteries and battery management for electric vehicles. Energy Rep. 2022, 8, 4058–4084. [Google Scholar] [CrossRef]
- Dai, H.; Jiang, B.; Hu, X.; Lin, X.; Wei, X.; Pecht, M. Advanced battery management strategies for a sustainable energy future: Multilayer design concepts and research trends. Renew. Sustain. Energy Rev. 2021, 138, 110480. [Google Scholar] [CrossRef]
- Feng, X.; Ren, D.; He, X.; Ouyang, M. Mitigating Thermal Runaway of Lithium-Ion Batteries. Joule 2020, 4, 743–770. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Qi, X.; Sidi, M.O.; Tlili, I.; Ibrahim, T.K.; Elkotb, M.A.; El-Shorbagy, M.A.; Li, Z. Optimization and sensitivity analysis of extended surfaces during melting and freezing of phase changing materials in cylindrical Lithium-ion battery cooling. J. Energy Storage 2022, 51, 104545. [Google Scholar] [CrossRef]
- Mehrabi-Kermani, M.; Houshfar, E.; Ashjaee, M. A novel hybrid thermal management for Li-ion batteries using phase change materials embedded in copper foams combined with forced-air convection. Int. J. Therm. Sci. 2019, 141, 47–61. [Google Scholar] [CrossRef]
- Kong, D.; Peng, R.; Ping, P.; Du, J.; Chen, G.; Wen, J. A novel battery thermal management system coupling with PCM and optimized controllable liquid cooling for different ambient temperatures. Energy Convers. Manag. 2020, 204, 112280. [Google Scholar] [CrossRef]
- Chen, K.; Hou, J.; Song, M.; Wang, S.; Wu, W.; Zhang, Y. Design of battery thermal management system based on phase change material and heat pipe. Appl. Therm. Eng. 2021, 188, 116665. [Google Scholar] [CrossRef]
- Chen, F.; Huang, R.; Wang, C.; Yu, X.; Liu, H.; Wu, Q.; Qian, K.; Bhagat, R. Air and PCM cooling for battery thermal management considering battery cycle life. Appl. Therm. Eng. 2020, 173, 115154. [Google Scholar] [CrossRef]
- Weng, J.; Huang, Q.; Li, X.; Zhang, G.; Ouyang, D.; Chen, M.; Yuen, A.C.Y.; Li, A.; Lee, E.W.M.; Yang, W.; et al. Safety Issue on PCM-based Battery Thermal Management: Material Thermal Stability and System Hazard Mitigation. Energy Storage Mater. 2022, 53, 580–612. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Zhu, C.; Wang, F.; Zhao, D.; Li, Z.; Liu, Y.; Zhang, H.; Jiang, H. Experimental investigation on the effect of phase change materials for thermal management improvement of the fast charging power module. Case Stud. Therm. Eng. 2023, 42, 102711. [Google Scholar] [CrossRef]
- Heyhat, M.M.; Mousavi, S.; Siavashi, M. Battery thermal management with thermal energy storage composites of PCM, metal foam, fin and nanoparticle. J. Energy Storage 2020, 28, 101235. [Google Scholar] [CrossRef]
- Jiang, G.; Huang, J.; Fu, Y.; Cao, M.; Liu, M. Thermal optimization of composite phase change material/expanded graphite for Li-ion battery thermal management. Appl. Therm. Eng. 2016, 108, 1119–1125. [Google Scholar] [CrossRef]
- Li, J.; Tang, A.; Shao, X.; Jin, Y.; Chen, W.; Xia, D. Experimental evaluation of heat conduction enhancement and lithium-ion battery cooling performance based on h-BN-based composite phase change materials. Int. J. Heat Mass Transf. 2022, 186, 122487. [Google Scholar] [CrossRef]
- Wu, W.; Yang, X.; Zhang, G.; Ke, X.; Wang, Z.; Situ, W.; Li, X.; Zhang, J. An experimental study of thermal management system using copper mesh-enhanced composite phase change materials for power battery pack. Energy 2016, 113, 909–916. [Google Scholar] [CrossRef]
- Zou, D.; Ma, X.; Liu, X.; Zheng, P.; Hu, Y. Thermal performance enhancement of composite phase change materials (PCM) using graphene and carbon nanotubes as additives for the potential application in lithium-ion power battery. Int. J. Heat Mass Transf. 2018, 120, 33–41. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Dong, W.; Wang, J.; Huang, X.; Wang, J.; Gao, H.; Wang, G. Synthesis and Characterization of Paraffin/Metal Organic Gel Derived Porous Carbon/Boron Nitride Composite Phase Change Materials for Thermal Energy Storage. Eur. J. Inorg. Chem. 2018, 48, 5167–5175. [Google Scholar] [CrossRef]
- Xiao, C.; Wu, X.; Dong, X.; Ye, G.; Zhang, G.; Yang, X. Ultrareliable Composite Phase Change Material for Battery Thermal Management Derived from a Rationally Designed Phase Changeable and Hydrophobic Polymer Skeleton. ACS Appl. Energy Mater. 2021, 4, 3832–3841. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, J.; Cao, M.; Liu, Z.; Chen, Q. A novel flexible phase change material with well thermal and mechanical properties for lithium batteries application. J. Energy Storage 2021, 44, 103433. [Google Scholar] [CrossRef]
- Hu, S.; Wang, S.; Zhang, Y.; Ma, C.; Wu, S.; Li, L. Effect of passive thermal management system on the electro-thermal performance of battery module. Int. J. Therm. Sci. 2023, 183, 107842. [Google Scholar] [CrossRef]
- Dai, X.; Kong, D.; Du, J.; Zhang, Y.; Ping, P. Investigation on effect of phase change material on the thermal runaway of lithium-ion battery and exploration of flame retardancy improvement. Process Saf. Environ. Prot. 2022, 159, 232–242. [Google Scholar] [CrossRef]
- Huang, Q.; Li, X.; Zhang, G.; Weng, J.; Wang, Y.; Deng, J. Innovative thermal management and thermal runaway suppression for battery module with flame retardant flexible composite phase change material. J. Clean. Prod. 2022, 330, 129718. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, Y.; Song, L.; Ni, J.; Xing, W.; Wang, J. Effect of expanded graphite on properties of high-density polyethylene/paraffin composite with intumescent flame retardant as a shape-stabilized phase change material. Sol. Energy Mater. Sol. Cells 2010, 94, 360–365. [Google Scholar] [CrossRef]
- Sittisart, P.; Farid, M.M. Fire retardants for phase change materials. Appl. Energy 2011, 88, 3140–3145. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Zhang, G.; Wu, H.; Rao, Z.; Guo, J.; Zhou, D. Experimental investigation of the flame retardant and form-stable composite phase change materials for a power battery thermal management system. J. Power Sources 2020, 480, 229116. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.; Li, X.; Zhang, G.; Chen, K.; Yang, W. Experimental investigation on thermal management system with flame retardant flexible phase change material for retired battery module. Appl. Energy 2022, 327, 120109. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, W.; Wu, T.; Wang, C.; Liang, Z. Thermal management system study of flame retardant solid–solid phase change material battery. Surf. Interfaces 2023, 36, 102558. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, X.; Ji, J.; Liu, L.; Wu, Y.; Yang, M.; Lu, D.; Zheng, H. Development of flexible form-stable phase change material with enhanced electrical resistance for thermal management. J. Clean. Prod. 2021, 311, 127517. [Google Scholar] [CrossRef]
- Liao, H.; Duan, W.; Liu, Y.; Wang, Q.; Wen, H. Flame retardant and leaking preventable phase change materials for thermal energy storage and thermal regulation. J. Energy Storage 2021, 35, 102248. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, H.; Zuo, H.; Zuo, Q.; He, X.; Chen, W.; Wei, R. EG@Bi-MOF derived porous carbon/lauric acid composite phase change materials for thermal management of batteries. Energy 2023, 272, 127180. [Google Scholar] [CrossRef]
- Song, G.; Ma, S.; Tang, G.; Yin, Z.; Wang, X. Preparation and characterization of flame retardant form-stable phase change materials composed by EPDM, paraffin and nano magnesium hydroxide. Energy 2010, 35, 2179–2183. [Google Scholar] [CrossRef]
- Kempel, F.; Schartel, B.; Marti, J.M.; Butler, K.M.; Rossi, R.; Idelsohn, S.R.; Onate, E.; Hofmann, A. Modelling the vertical UL 94 test: Competition and collaboration between melt dripping, gasification and combustion. Fire Mater. 2015, 39, 570–584. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Z.; Su, S.; Wu, H.; Sun, M.; Tang, L. Synergistic flame retardancy of ZnO and piperazine pyrophosphate/melamine cyanurate in polypropylene. J. Vinyl Addit. Technol. 2023, 29, 202–219. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Xu, Y.; Wang, C.; Chu, F. Effect of zinc oxide on properties of phenolic foams/halogen-free flame retardant system. J. Appl. Polym. Sci. 2015, 132, 42730. [Google Scholar] [CrossRef]
- Wu, W.; Liu, J.; Liu, M.; Rao, Z.; Deng, H.; Wang, Q.; Qi, X.; Wang, S. An innovative battery thermal management with thermally induced flexible phase change material. Energy Convers. Manag. 2020, 221, 113145. [Google Scholar] [CrossRef]
- Pradeep, G.M.; Sankaramoorthy, T.; Elango, M.; Kumar, T.N.; Girimurugan, R. Structural analysis and mechanical properties of thermal battery by flexible phase change materials [P.C.M.]. Mater. Today Proc. 2021, 56, 3196–3200. [Google Scholar] [CrossRef]












| Sample | Mass Content (wt%) | |||
|---|---|---|---|---|
| PA | HDPE | EG | FR | |
| PA | 100 | 0 | 0 | 0 |
| CPCM15 | 60 | 20 | 5 | 15 |
| CPCM20 | 55 | 20 | 5 | 20 |
| CPCM25 | 50 | 20 | 5 | 25 |
| CPCM30 | 45 | 20 | 5 | 30 |
| PA | CPCM15 | CPCM20 | CPCM25 | CPCM30 | |
|---|---|---|---|---|---|
| UL-94 | NR | NR | NR | V-0 | NR |
| LOI | 17.7% | 22.4% | 23.8% | 28.5% | 26.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Zhang, J.; Zhu, M.; Zhao, L.; Chen, Y.; Chen, M. Experimental Investigation on the Thermal Management for Lithium-Ion Batteries Based on the Novel Flame Retardant Composite Phase Change Materials. Batteries 2023, 9, 378. https://doi.org/10.3390/batteries9070378
Yu Y, Zhang J, Zhu M, Zhao L, Chen Y, Chen M. Experimental Investigation on the Thermal Management for Lithium-Ion Batteries Based on the Novel Flame Retardant Composite Phase Change Materials. Batteries. 2023; 9(7):378. https://doi.org/10.3390/batteries9070378
Chicago/Turabian StyleYu, Yue, Jiaxin Zhang, Minghao Zhu, Luyao Zhao, Yin Chen, and Mingyi Chen. 2023. "Experimental Investigation on the Thermal Management for Lithium-Ion Batteries Based on the Novel Flame Retardant Composite Phase Change Materials" Batteries 9, no. 7: 378. https://doi.org/10.3390/batteries9070378