Controllable Preparation to Boost High Performance of Nanotubular SiO2@C as Anode Materials for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Fabrication of Nanotubular SiO2@C Composites
2.3. Characterizations
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Structure and Morphology Analysis
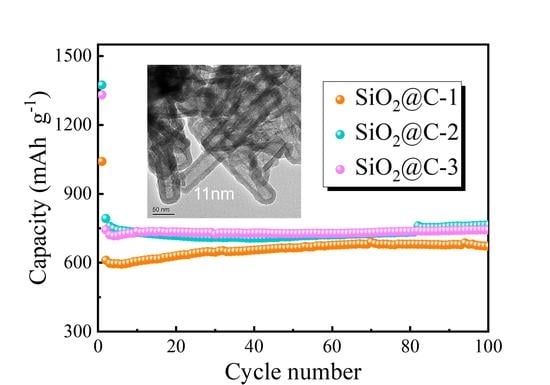
3.2. Electrochemical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- McDowell, M.T.; Lee, S.W.; Nix, W.D.; Cui, Y. 25th Anniversary Article: Understanding the Lithiation of Silicon and Other Alloying Anodes for Lithium-Ion Batteries. Adv. Mater. 2013, 25, 4966–4985. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.B.; Yu, S.W.; Yao, L.F.; Li, F.S.; Li, X.; Cheng, F.X.; Shen, X.; Sun, C.K.; Guo, H.; Liu, J.J. Robust hexagonal nut-shaped titanium(IV) MOF with porous structure for ultra-high performance lithium storage. Electrochim. Acta 2019, 296, 746–754. [Google Scholar] [CrossRef]
- Huang, G.Y.; Xu, S.M.; Xu, Z.H.; Sun, H.Y.; Li, L.Y. Core-Shell Ellipsoidal MnCo2O4 Anode with Micro–/Nano–Structure and Concentration Gradient for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 21325–21334. [Google Scholar] [CrossRef]
- Guo, T.; Luo, G.Y.; Shi, C.Y.; Shi, H.L.; Shi, Z.X.; He, B.F.; Chen, J.B. Thermal Burst Synthesis of High-Performance Si Nanotube Sheets for Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2022, 10, 4031–4039. [Google Scholar] [CrossRef]
- Yoo, G.W.; Kim, C.; Jang, B.C.; Yang, S.B.; Son, J.T. Synthesis of Hollow Nanorods of SiO2 Anode Material by AAO Template Synthesis Method for Lithium Ion Battery. J. Nanosci. Nanotechnol. 2015, 15, 8773–8776. [Google Scholar] [CrossRef] [PubMed]
- Lener, G.; Otero, M.; Barraco, D.E.; Leiva, E.P.M. Energetics of silica lithiation and its applications to lithium ion batteries. Electrochim. Acta 2017, 259, 1053–1058. [Google Scholar] [CrossRef]
- Li, W.; Wang, F.; Ma, M.N.; Zhou, J.S.; Liu, Y.W.; Chen, Y. Preparation of SiO2 nanowire arrays as anode material with enhanced lithium storage performance. RSC Adv. 2018, 8, 33652–33658. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Q.; Huang, H.; Li, Z.H.; Zhong, M.L.; Zhang, G.Q.; Wu, D.C. Preparation and lithium-storage performance of carbon/silica composite with a unique porous bicontinuous nanostructure. Carbon 2014, 77, 275–280. [Google Scholar] [CrossRef]
- Takezawa, H.; Iwamoto, K.; Ito, S.; Yoshizawa, H. Electrochemical behaviors of nonstoichiometric silicon suboxides (SiOx) film prepared by reactive evaporation for lithium rechargeable batteries. J. Power Sources 2013, 244, 149–157. [Google Scholar] [CrossRef]
- Luo, F.; Li, X.C.; He, G.H.; Li, M.; Zhang, H.L. Preparation of Double-Shelled C/SiO2 Hollow Spheres with Enhanced Adsorption Capacity. Ind. Eng. Chem. Res. 2015, 54, 641–648. [Google Scholar] [CrossRef]
- Hou, X.H.; Zhang, M.; Wang, J.Y.; Hu, S.J.; Liu, X.; Shao, Z.P. High yield and low-cost ball milling synthesis of nano-flake Si@SiO2 with small crystalline grains and abundant grain boundaries as a superior anode for Li-ion batteries. J. Alloys Compd. 2015, 639, 27–35. [Google Scholar] [CrossRef]
- Chang, W.S.; Park, C.M.; Kim, J.H.; Kim, Y.U.; Jeong, G.; Sohn, H.J. Quartz (SiO2): A new energy storage anode material for Li-ion batteries. Energy Environ. Sci. 2012, 5, 6895–6899. [Google Scholar] [CrossRef]
- Dirican, M.; Lu, Y.; Fu, K.; Kizil, H.; Zhang, X.W. SiO2-confined silicon/carbon nanofiber composites as an anode for lithium-ion batteries. RSC Adv. 2015, 5, 34744–34751. [Google Scholar] [CrossRef]
- Liu, X.L.; Chen, Y.X.; Liu, H.B.; Liu, Z.Q. SiO2@C hollow sphere anodes for lithium-ion batteries. J. Mater. Sci. Technol. 2016, 33, 239–245. [Google Scholar] [CrossRef]
- Cao, X.; Chuan, X.Y.; Li, S.; Huang, D.B.; Cao, G.Z. Hollow Silica Spheres Embedded in a Porous Carbon Matrix and Its Superior Performance as the Anode for Lithium-Ion Batteries. Part. Part. Syst. Char. 2016, 33, 110–117. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.L.; Liu, G.; Mcilwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef]
- Favors, Z.; Wang, W.; Bay, H.H.; George, A.; Ozkan, M.; Ozkan, C.S. Stable Cycling of SiO2 Nanotubes as High-Performance Anodes for Lithium-Ion Batteries. Sci. Rep. 2014, 4, 4605. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.L.; Liang, D.W.; Zhao, H.D. Enhanced Electrochemical Performance Promoted by Tin in Silica Anode Materials for Stable and High-Capacity Lithium-Ion Batteries. Materials 2021, 5, 1071. [Google Scholar] [CrossRef]
- Ding, X.L.; Liang, D.W.; Ai, X.; Zhao, H.D.; Zhao, N.; Chen, X.J.; Xu, J.H.; Yang, H. Synergistic Lithium Storage in Silica-Tin Composites Enables a Cycle-Stable and High-Capacity Anode for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 2741–2750. [Google Scholar] [CrossRef]
- Jia, D.L.; Wang, K.; Huang, J.G. Filter paper derived nanofibrous silica-carbon composite as anodic material with enhanced lithium storage performance. Chem. Eng. J. 2017, 317, 673–686. [Google Scholar] [CrossRef]
- Wang, C.W.; Liu, K.W.; Chen, W.F.; Zhou, J.D.; Lin, H.P.; Hsu, C.H.; Kuo, P.L. Mesoporous SiO2/carbon hollow spheres applied towards a high rate-performance Li-battery anode. Inorg. Chem. Front. 2016, 3, 1398–1405. [Google Scholar] [CrossRef]
- Xiao, T.T.; Zhang, W.F.; Xu, T.; Wu, J.X.; Wei, M.D. Hollow SiO2 microspheres coated with nitrogen doped carbon layer as an anode for high performance lithium-ion batteries. Electrochim. Acta 2019, 306, 106–112. [Google Scholar] [CrossRef]
- Yuan, Z.N.; Zhao, N.Q.; Shi, C.S.; Liu, E.; He, C.N.; He, F. Synthesis of SiO2/3D porous carbon composite as anode material with enhanced lithium storage performance. Chem. Phys. Lett. 2016, 651, 19–23. [Google Scholar] [CrossRef]
- Guo, B.K.; Shu, J.; Wang, Z.X.; Yang, H.; Shi, L.H.; Liu, Y.N.; Chen, L.Q. Electrochemical reduction of nano-SiO2 in hard carbon as anode material for lithium ion batteries. Electrochem. Commun. 2008, 10, 1876–1878. [Google Scholar] [CrossRef]
- Lu, T.H.; Wang, H.; Wu, P.; Qu, M.T.; Si, L.; Tang, Y.W.; Zhou, Y.M. Highly Reversible and Fast Lithium Storage in Graphene Wrapped SiO2 Nanotube Network. Nanoscale 2012, 4, 4002–4006. [Google Scholar] [CrossRef]
- An, W.L.; Fu, J.J.; Su, J.J.; Wang, L.; Peng, X.; Wu, K.M.; Chen, Q.Y.; Bi, Y.J.; Gao, B.; Zhang, X.M. Mesoporous hollow nanospheres consisting of carbon coated silica nanoparticles for robust lithium-ion battery anodes. J. Power Sources 2017, 345, 227–236. [Google Scholar] [CrossRef]
- Chen, Y.F.; Du, N.; Zhang, H.; Yang, D.R. Porous Si@C coaxial nanotubes: Layer-by-layer assembly on ZnO nanorod templates and application to lithium-ion batteries. CrystEngComm 2017, 19, 1220–1229. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, Y.J. Improving the Flame Retardancy and Smoke Suppression of Poly(Lactic Acid) with a SiO2@ammonium Molybdate Core-Shell Nanotubes. Polym.-Plast. Technol. Eng. 2019, 58, 843–853. [Google Scholar] [CrossRef]
- Ren, Y.R.; Yang, B.; Wei, H.M.; Ding, J.N. Electrospun SiO2/C composite fibers as durable anode materials for lithium ion batteries. Solid State Ion. 2016, 292, 27–31. [Google Scholar] [CrossRef]
- Zheng, J.P.; Yu, Z.Z.; Wang, X.; Su, Q.; Shan, J.H. The effect of silica nanotubes on mechanical performance of polymethyl methacrylate nanocomposites: Comparison to spherical nano-silica. J. Reinf. Plast. Compos. 2015, 34, 1433–1443. [Google Scholar] [CrossRef]
- Zhang, X.; Li, K.; Li, Y.B.; Liu, J.J.; Dai, J.T.; Li, Y.F.; Ai, F.R. Facile fabrication of SiO2 nanotubes coated with nitrogen-doped carbon layers as high-performance anodes for lithium-ion batteries. Ceram. Int. 2020, 47, 1373–1380. [Google Scholar] [CrossRef]
- Shao, D.; Tang, D.P.; Mai, Y.J.; Zhang, L.Z. Nanostructured silicon/porous carbon spherical composite as a high capacity anode for Li-ion batteries. J. Mater. Chem. A 2013, 1, 15068–15075. [Google Scholar] [CrossRef]
- Agger, J.R.; Anderson, M.W.; Pemble, M.E.; Terasaki, O.; Nozue, Y. Growth of Quantum-Confined Indium Phosphide inside MCM-41. J. Phys. Chem. B 1998, 102, 3345–3353. [Google Scholar] [CrossRef]
- Fan, W.G.; Gao, L. Synthesis of silicon dioxide hollow spheres assisted by ultrasound. J. Colloid Interface Sci. 2006, 297, 157–160. [Google Scholar] [CrossRef]
- Alam, M.M.; Yamahana, H.; Bastakoti, B.P.; Luitel, H.N.; Zhao, W.W.; Yamauchi, Y.; Watari, T.; Noguchi, H.; Nakashima, K. Synthesis of hollow silica nanosphere with high accessible surface area and their hybridization with carbon matrix for drastic enhancement of electrochemical property. Appl. Surf. Sci. 2014, 314, 552–557. [Google Scholar] [CrossRef]
- Liu, H.T.; Shan, Z.Q.; Huang, W.L.; Wang, D.D.; Lin, Z.J.; Cao, Z.J.; Chen, P.; Meng, S.X.; Chen, L. Self-Assembly of Silicon@Oxidized Mesocarbon Microbeads Encapsulated in Carbon as Anode Material for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 4715–4725. [Google Scholar] [CrossRef]
- Sen, P.N.; Thorpe, M.F. Phonons in AX2 glasses: From molecular to band-like modes. Phys. Rev. B 1977, 15, 4030–4038. [Google Scholar] [CrossRef]
- Ma, B.; Lu, B.; Luo, J.; Deng, X.; Wu, Z.; Wang, X. The hollow mesoporous silicon nanobox dually encapsulated by SnO2/C as anode material of lithium ion battery. Electrochi. Acta 2018, 288, 61–70. [Google Scholar] [CrossRef]
- Ma, X.M.; Wei, Z.P.; Han, H.J.; Wang, X.B.; Cui, K.Q.; Yang, L. Tunable construction of multi-shell hollow SiO2 microspheres with hierarchically porous structure as high-performance anodes for lithium-ion batteries. Chem. Eng. J. 2017, 323, 252–259. [Google Scholar] [CrossRef]
- Tang, C.J.; Liu, Y.N.; Xu, C.; Zhu, J.X.; Wei, X.J.; Zhou, L.; He, L.; Yang, W.; Mai, L.Q. Ultrafine Nickel-Nanoparticle-Enabled SiO2 Hierarchical Hollow Spheres for High-Performance Lithium Storage. Adv. Funct. Mater. 2017, 28, 1704561. [Google Scholar] [CrossRef]
- Liang, Y.H.; Chen, Y.M.; Ke, X.; Zhang, Z.X.; Wu, W.L.; Lin, G.D.; Zhou, Z.; Shi, Z.C. Coupling of Triporosity and Strong Au-Li Interaction to Enable Dendrite-Free Lithium Plating/Stripping for Long-Life Lithium Metal Anodes. J. Mater. Chem. A 2020, 8, 18094–18105. [Google Scholar] [CrossRef]
- Li, M.; Li, J.H.; Li, K.; Zhao, Y.; Zhang, Y.G.; Gosselink, D.; Chen, P. SiO2/Cu/polyacrylonitrile-C composite as anode material in lithium ion batteries. J. Power Sources 2013, 240, 659–666. [Google Scholar] [CrossRef]
- Lu, B.; Ma, B.J.; Deng, X.L.; Wu, B.; Wu, Z.Y.; Luo, J.; Wang, X.Y.; Chen, G.R. Dual stabilized architecture of hollow Si@TiO2@C nanospheres as anode of high-performance Li-ion battery. Chem. Eng. J. 2018, 351, 269–279. [Google Scholar] [CrossRef]
- Liu, G.P.; Wang, N.; Qi, F.Y.; Lu, X.Y.; Liang, Y.H.; Sun, Z.P. Novel Ni-Ge-P anodes for lithium-ion batteries with enhanced reversibility and reduced redox potential. Inorg. Chem. Front. 2022, 10, 699–711. [Google Scholar] [CrossRef]
- Tang, J.; Dai, X.Y.; Wu, F.Z.; Ma, Y.; Wang, X.; Jin, H.X.; Gu, Y.J.; Xie, Y.F. A simple and efficient one-pot synthesis of SiO2 nanotubes with stable structure and controlled aspect ratios for anode materials of lithium-ion batteries. Ionics 2019, 26, 639–648. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, X.X.; Tu, K.K.; Liu, D.; Tang, H.L.; Li, J.S.; Li, X.; Xie, Z.Z.; Qu, D.Y. Synthesis of Carbon-SiO2 hybrid layer@SiO2@CNT coaxial nanotube and its application in lithium storage. Electrochim. Acta 2020, 354, 136726. [Google Scholar] [CrossRef]
- Wang, K.X.; Zhu, X.D.; Hu, Y.J.; Qiu, S.Y.; Gu, L.L.; Wang, C.; Zuo, P.J. Stable anchoring and uniform distribution of SiO2 nanotubes on reduced graphene oxide through electrostatic self-assembly for ultra-high lithium storage performance. Carbon 2020, 167, 835–842. [Google Scholar] [CrossRef]
- Wang, S.Q.; Zhao, N.Q.; Shi, C.S.; Liu, E.Z.; He, C.N.; He, F.; Mam, L.Y. In-situ grown CNTs modified SiO2/C composites as anode with improved cycling stability and rate capability for lithium storage. Appl. Surf. Sci. 2018, 433, 428–436. [Google Scholar] [CrossRef]








| Materials | Active Material Loading (mg cm−2) | Current Density (A g−1)/Capacity (mAh g−1)/Cycle Number (n) | Current Density (A g−1)/Capacity (mAh g−1)/Cycle Number (n) | Ref. |
|---|---|---|---|---|
| SiO2@C composites | 0.26–0.41 | 0.2/759.1/100 | 1/500/526.3 | this work |
| SNTs@NC | - | 0.1/781/200 | - | [32] |
| SiO2 nanotubes | - | 0.04/232.5/100 | - | [46] |
| SiO2/C@SiO2@CNT | 1.0 | 0.1/644/200 | 1/5000/242 | [47] |
| SiO2/rGO | 1.0 | 0.2/961/250 | 1/800/801 | [48] |
| SiO2/C/CNT composites | 0.87 | 0.05/502.3/100 | 1/315.7/1000 | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, C.; Chen, J.; Guo, T.; Luo, G.; Shi, H.; Shi, Z.; Qin, G.; Zhang, L.; He, X. Controllable Preparation to Boost High Performance of Nanotubular SiO2@C as Anode Materials for Lithium-Ion Batteries. Batteries 2023, 9, 107. https://doi.org/10.3390/batteries9020107
Shi C, Chen J, Guo T, Luo G, Shi H, Shi Z, Qin G, Zhang L, He X. Controllable Preparation to Boost High Performance of Nanotubular SiO2@C as Anode Materials for Lithium-Ion Batteries. Batteries. 2023; 9(2):107. https://doi.org/10.3390/batteries9020107
Chicago/Turabian StyleShi, Chaoyun, Jingbo Chen, Tong Guo, Guiyang Luo, Huili Shi, Zixu Shi, Guoqiang Qin, Long Zhang, and Xiangming He. 2023. "Controllable Preparation to Boost High Performance of Nanotubular SiO2@C as Anode Materials for Lithium-Ion Batteries" Batteries 9, no. 2: 107. https://doi.org/10.3390/batteries9020107