Improving the Structural Ordering and Particle-Size Homogeneity of Li-Rich Layered Li1.2Ni0.13Co0.13Mn0.54O2 Cathode Materials through Microwave Irradiation Solid-State Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of LNCM
2.2. Materials Characterizations
2.3. Electrochemical Properties Characterizations
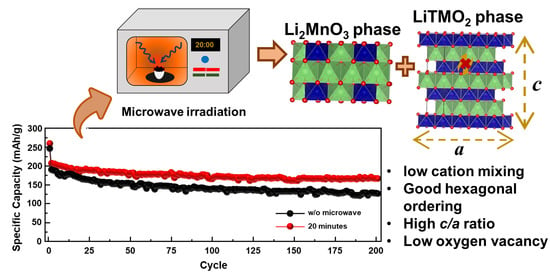
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmad, F.; Khalid, M.; Ketan, B. Development in Energy Storage System for Electric Transportation: A Comprehensive Review. J. Energy Storage 2021, 43, 103153. [Google Scholar] [CrossRef]
- Eglitis, R.I.; Borstel, G. Towards a Practical Rechargeable 5 V Li Ion Battery. Phys. Status Solidi Appl. Mater. Sci. 2005, 202, 13–15. [Google Scholar] [CrossRef]
- Eglitis, R.I. Theoretical Prediction of the 5 V Rechargeable Li Ion Battery Using Li2CoMn3O8 as a Cathode. Phys. Scr. 2015, 90, 94012. [Google Scholar] [CrossRef]
- Ji, X.; Xia, Q.; Xu, Y.; Feng, H.; Wang, P.; Tan, Q. A Review on Progress of Lithium-Rich Manganese-Based Cathodes for Lithium Ion Batteries. J. Power Sources 2021, 487, 229362. [Google Scholar] [CrossRef]
- Zuo, W.; Luo, M.; Liu, X.; Wu, J.; Liu, H.; Li, J.; Winter, M.; Fu, R.; Yang, W.; Yang, Y. Li-Rich Cathodes for Rechargeable Li-Based Batteries: Reaction Mechanisms and Advanced Characterization Techniques. Energy Environ. Sci. 2020, 13, 4450–4497. [Google Scholar] [CrossRef]
- Zheng, J.; Myeong, S.; Cho, W.; Yan, P.; Xiao, J.; Wang, C.; Cho, J.; Zhang, J.G. Li- and Mn-Rich Cathode Materials: Challenges to Commercialization. Adv. Energy Mater. 2017, 7, 1601284. [Google Scholar] [CrossRef]
- Zheng, H.; Han, X.; Guo, W.; Lin, L.; Xie, Q.; Liu, P.; He, W.; Wang, L.; Peng, D.L. Recent Developments and Challenges of Li-Rich Mn-Based Cathode Materials for High-Energy Lithium-Ion Batteries. Mater. Today Energy 2020, 18, 100518. [Google Scholar] [CrossRef]
- Koga, H.; Croguennec, L.; Ménétrier, M.; Mannessiez, P.; Weill, F.; Delmas, C. Different Oxygen Redox Participation for Bulk and Surface: A Possible Global Explanation for the Cycling Mechanism of Li1.20Mn0.54Co0.13Ni0.13O2. J. Power Sources 2013, 236, 250–258. [Google Scholar] [CrossRef]
- Zhao, T.; Zhou, N.; Zhang, X.; Xue, Q.; Wang, Y.; Yang, M.; Li, L.; Chen, R. Structure Evolution from Layered to Spinel during Synthetic Control and Cycling Process of Fe-Containing Li-Rich Cathode Materials for Lithium-Ion Batteries. ACS Omega 2017, 2, 5601–5610. [Google Scholar] [CrossRef]
- Wei, W.; Chen, L.; Pan, A.; Ivey, D.G. Roles of Surface Structure and Chemistry on Electrochemical Processes In Lithium-Rich Layered Oxide Cathodes. Nano Energy 2016, 30, 580–602. [Google Scholar] [CrossRef]
- Chen, Z.; Meng, J.; Wang, Y.; Ma, Q.; Lai, F.; Li, Z.; Zhang, Q.; Li, D.; Zhong, S. Local Structure Modulation via Cation Compositional Regulation for Durable Li-Rich Layered Cathode Materials. Electrochim. Acta 2021, 378, 138138. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, W.J.; Mauger, A.; Qilu; Gendron, F.; Julien, C.M. Minimization of the Cation Mixing in Li1+x(NMC)1-xO2 as Cathode Material. J. Power Sources 2010, 195, 1292–1301. [Google Scholar] [CrossRef]
- Yan, W.; Xie, Y.; Jiang, J.; Sun, D.; Ma, X.; Lan, Z.; Jin, Y. Enhanced Rate Performance of Al-Doped Li-Rich Layered Cathode Material via Nucleation and Post-Solvothermal Method. ACS Sustain. Chem. Eng. 2018, 6, 4625–4632. [Google Scholar] [CrossRef]
- Sun, G.; Yin, X.; Yang, W.; Song, A.; Jia, C.; Yang, W.; Du, Q.; Ma, Z.; Shao, G. The Effect of Cation Mixing Controlled by Thermal Treatment Duration on the Electrochemical Stability of Lithium Transition-Metal Oxides. Phys. Chem. Chem. Phys. 2017, 19, 29886–29894. [Google Scholar] [CrossRef]
- Cui, S.L.; Gao, M.Y.; Li, G.R.; Gao, X.P. Insights into Li-Rich Mn-Based Cathode Materials with High Capacity: From Dimension to Lattice to Atom. Adv. Energy Mater. 2022, 12, 2003885. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Z.; Shen, X.; Li, W.; Gao, Y.; Banis, M.N.; Li, M.; Chen, K.; Zhu, L.; Yu, R.; et al. Surface Doping to Enhance Structural Integrity and Performance of Li-Rich Layered Oxide. Adv. Energy Mater. 2018, 8, 1802105. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Li, Y.; Xi, X.; Zheng, J.; Zhu, Y.; Xiong, Y.; Liu, S. Encouraging Voltage Stability upon Long Cycling of Li-Rich Mn-Based Cathode Materials by Ta-Mo Dual Doping. ACS Appl. Mater. Interfaces 2021, 13, 25981–25992. [Google Scholar] [CrossRef]
- Kim, S.; Cho, W.; Zhang, X.; Oshima, Y.; Choi, J.W. A Stable Lithium-Rich Surface Structure for Lithium-Rich Layered Cathode Materials. Nat. Commun. 2016, 7, 13598. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Liu, Y.; Zhang, Y.; Xing, G.; Tang, Y. Lithium-Rich Sulfide/Selenide Cathodes for next-Generation Lithium-Ion Batteries: Challenges and Perspectives. Chem. Commun. 2022, 58, 3591–3600. [Google Scholar] [CrossRef]
- Lin, T.; Schulli, T.U.; Hu, Y.; Zhu, X.; Gu, Q.; Luo, B.; Cowie, B.; Wang, L. Faster Activation and Slower Capacity/Voltage Fading: A Bifunctional Urea Treatment on Lithium-Rich Cathode Materials. Adv. Funct. Mater. 2020, 30, 1909192. [Google Scholar] [CrossRef]
- Karunawan, J.; Irham, M.A.; Widyadharma, P.H.; Floweri, O.; Aimon, A.H.; Iskandar, F. Effect of NaTi2(PO4)3 Coating on Improving Capacity Retention of Li-Rich. In Proceedings of the 2019 6th International Conference on Electric Vehicular Technology (ICEVT), Bali, Indonesia, 18–21 November 2019; pp. 11–13. [Google Scholar] [CrossRef]
- Pimenta, V.; Sathiya, M.; Batuk, D.; Abakumov, A.M.; Giaume, D.; Cassaignon, S.; Larcher, D.; Tarascon, J.M. Synthesis of Li-Rich NMC: A Comprehensive Study. Chem. Mater. 2017, 29, 9923–9936. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Z.; Chen, M.; Zou, C.; Jin, H.; Wang, S.; Chou, S.L.; Liu, Y.; Dou, S.X. The Cathode Choice for Commercialization of Sodium-Ion Batteries: Layered Transition Metal Oxides versus Prussian Blue Analogs. Adv. Funct. Mater. 2020, 30, 1909530. [Google Scholar] [CrossRef]
- Zhou, L.Z.; Xu, Q.J.; Liu, M.S.; Jin, X. Novel Solid-State Preparation and Electrochemical Properties of Li1.13[Ni0.2Co0.2Mn0.47]O2 with a High Capacity by Acetate Precursor for Li-ion Batteries. Solid State Ion. 2013, 249–250, 134–138. [Google Scholar] [CrossRef]
- Hao, W.; Zhan, H.; Chen, H.; Wang, Y.; Tan, Q.; Su, F. Solid-State Synthesis of Li[Li0.2Mn0.56Ni0.16Co0.08]O2 Cathode Materials for Lithium-Ion Batteries. Particuology 2014, 15, 18–26. [Google Scholar] [CrossRef]
- Du, C.Q.; Zhang, F.; Ma, C.X.; Wu, J.W.; Tang, Z.Y.; Zhang, X.H.; Qu, D. Synthesis and Electrochemical Properties of Li1.2Mn0.54Ni0.13Co0.13O2 Cathode Material for Lithium-Ion Battery. Ionics 2016, 22, 209–218. [Google Scholar] [CrossRef]
- Haruna, A.B.; Ozoemena, K.I. Effects of Microwave Irradiation on the Electrochemical Performance of Manganese-Based Cathode Materials for Lithium-Ion Batteries. Curr. Opin. Electrochem. 2019, 18, 16–23. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, Z.; Zhang, Y.; Du, P.; Chen, H. Ultrasonic/Microwave-Assisted Co-Precipitation Method in the Synthesis of Li1.1Mn0.433Ni0.233Co0.233O2 Cathode Material for Lithium-Ion Batteries. Mater. Lett. 2014, 136, 160–163. [Google Scholar] [CrossRef]
- Miao, X.; Yan, Y.; Wang, C.; Cui, L.; Fang, J.; Yang, G. Optimal Microwave-Assisted Hydrothermal Synthesis of Nanosized xLi2MnO3·(1 − x) LiNi1/3Co1/3Mn1/3O2 Cathode Materials for Lithium Ion Battery. J. Power Sources 2014, 247, 219–227. [Google Scholar] [CrossRef]
- Shi, S.; Wang, T.; Cao, M.; Wang, J.; Zhao, M.; Yang, G. Rapid Self-Assembly Spherical Li1.2Mn0.56Ni0.16Co0.08O2 with Improved Performances by Microwave Hydrothermal Method as Cathode for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 11476–11487. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A Graphical User Interface for the Rietveld Refinement Program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [Green Version]
- Redel, K.; Kulka, A.; Plewa, A.; Molenda, J. High-Performance Li-Rich Layered Transition Metal Oxide Cathode Materials for Li-Ion Batteries. J. Electrochem. Soc. 2019, 166, A5333–A5342. [Google Scholar] [CrossRef]
- Lv, C.; Peng, Y.; Yang, J.; Duan, X.; Ma, J.; Wang, T. Electrospun Nb-Doped LiNi0.4Co0.2Mn0.4O2 Nanobelts for Lithium-Ion Batteries. Inorg. Chem. Front. 2018, 5, 1126–1132. [Google Scholar] [CrossRef]
- Karunawan, J.; Floweri, O.; Santosa, S.P.; Sumboja, A.; Iskandar, F. Stable Layered-Layered-Spinel Structure of the Li1.2Ni0.13Co0.13Mn0.54O2 Cathode Synthesized by Ball-Milling Assisted Solid-State Method. J. Electroanal. Chem. 2022, 907, 116050. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Croy, J.R.; Lee, E.; Gutierrez, A.; He, M.; Park, J.S.; Yonemoto, B.T.; Long, B.R.; Blauwkamp, J.D.; Johnson, C.S.; et al. The Quest for Manganese-Rich Electrodes for Lithium Batteries: Strategic Design and Electrochemical Behavior. Sustain. Energy Fuels 2018, 2, 1375–1397. [Google Scholar] [CrossRef]
- Chakraborty, A.; Kunnikuruvan, S.; Kumar, S.; Markovsky, B.; Aurbach, D.; Dixit, M.; Major, D.T. Layered Cathode Materials for Lithium-Ion Batteries: Review of Computational Studies on LiNi1–x–yCoxMnyO2 and LiNi1–x–yCoxAlyO2. Chem. Mater. 2020, 32, 915–952. [Google Scholar] [CrossRef]
- Yang, P.; Li, H.; Wei, X.; Zhang, S.; Xing, Y. Structure Tuned Li1.2Mn0.6Ni0.2O2 with Low Cation Mixing and Ni Segregation as High Performance Cathode Materials for Li-Ion Batteries. Electrochim. Acta 2018, 271, 276–283. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, N.; Zhao, L.; Xu, J.; Liu, Z.; Liu, Y.; Wu, J.; Ding, F. Improving Electrochemical Performance of Lithium-Rich Cathode Material Li1.2Mn0.52Ni0.13Co0.13W0.02O2 Coated with Li2WO4 for Lithium Ion Batteries. J. Alloys Compd. 2019, 811, 152023. [Google Scholar] [CrossRef]
- Hua, W.; Zhang, J.; Zheng, Z.; Liu, W.; Peng, X.; Guo, X.D.; Zhong, B.; Wang, Y.J.; Wang, X. Na-Doped Ni-Rich LiNi0.5Co0.2Mn0.3O2 Cathode Material with Both High Rate Capability and High Tap Density for Lithium Ion Batteries. Dalt. Trans. 2014, 43, 14824–14832. [Google Scholar] [CrossRef]
- Xie, Y.; Yin, J.; Chen, X.; Liang, X.; Jin, Y.; Xiang, L. Synergistic Effect of Mn3+ Formation-Migration and Oxygen Loss on the near Surface and Bulk Structural Changes in Single Crystalline Lithium-Rich Oxides. ACS Appl. Mater. Interfaces 2021, 13, 3891–3898. [Google Scholar] [CrossRef]
- Jafta, C.J.; Raju, K.; Mathe, M.K.; Manyala, N.; Ozoemena, K.I. Microwave Irradiation Controls the Manganese Oxidation States of Nanostructured (Li[Li0.2Mn0.52Ni0.13Co0.13Al0.02]O2) Layered Cathode Materials for High-Performance Lithium Ion Batteries. J. Electrochem. Soc. 2015, 162, A768–A773. [Google Scholar] [CrossRef] [Green Version]
- Jafta, C.J.; Mathe, M.K.; Manyala, N.; Roos, W.D.; Ozoemena, K.I. The Effect of Microwave Treatment on the Mn3+ Concentration of Spinel Cathode Material Studied By XPS, XRD and Electrochemical Method. In Proceedings of the 224th ECS Meeting, San Francisco, CA, USA, 27 October–1 November 2013; pp. 4–10. [Google Scholar]
- Susai, F.A.; Talianker, M.; Liu, J.; Rosy; Paul, T.; Grinblat, Y.; Erickson, E.; Noked, M.; Burstein, L.; Frenkel, A.I.; et al. Electrochemical Activation of Li2MnO3 Electrodes at 0 °C and Its Impact on the Subsequent Performance at Higher Temperatures. Materials 2020, 13, 4388. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Ding, X.; Fan, J.; Zhang, Z.; Liu, P.; Yang, X.; Guo, J.; Sun, S.; Lin, Z. Accurate Control of Initial Coulombic Efficiency for Lithium-Rich Manganese-Based Layered Oxides by Surface Multicomponent Integration. Angew. Chem.—Int. Ed. 2020, 59, 23061–23066. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Ma, J.; Jiang, N.; Xia, D. Effects of Particle Size on Voltage Fade for Li-Rich Mn-Based Layered Oxides. ACS Omega 2018, 3, 11136–11143. [Google Scholar] [CrossRef] [PubMed]
- Kaewmala, S.; Limphirat, W.; Yordsri, V.; Nash, J.; Srilomsak, S.; Kesorn, A.; Limthongkul, P.; Meethong, N. Rate Dependent Structural Changes, Cycling Stability, and Li-Ion Diffusivity in a Layered–Layered Oxide Cathode Material after Prolonged Cycling. J. Mater. Chem. A 2021, 9, 14004–14012. [Google Scholar] [CrossRef]
- Jossen, A. Encyclpedia of Electrochemical Power Sources. Batter. Dyn. 2009, 2, 478–488. [Google Scholar] [CrossRef]
- Song, J.H.; Yoon, G.; Kim, B.; Eum, D.; Park, H.; Kim, D.H.; Kang, K. Anionic Redox Activity Regulated by Transition Metal in Lithium-Rich Layered Oxides. Adv. Energy Mater. 2020, 10, 2001207. [Google Scholar] [CrossRef]
- Aang, Y.; Shang, K.; He, W.; Ai, X.; Cao, Y.; Yang, H. Magnesium-Doped Li1.2[Co0.13Ni0.13Mn0.54]O2 for Lithium-Ion Battery Cathode with Enhanced Cycling Stability and Rate Capability. ACS Appl. Mater. Interfaces 2015, 7, 13014–13021. [Google Scholar] [CrossRef]
- Wang, E.; Zhao, Y.; Xiao, D.; Zhang, X.; Wu, T.; Wang, B.; Zubair, M.; Li, Y.; Sun, X.; Yu, H. Composite Nanostructure Construction on the Grain Surface of Li-Rich Layered Oxides. Adv. Mater. 2020, 32, 1906070. [Google Scholar] [CrossRef]
- Zhang, X.D.; Shi, J.L.; Liang, J.Y.; Yin, Y.X.; Zhang, J.N.; Yu, X.Q.; Guo, Y.G. Suppressing Surface Lattice Oxygen Release of Li-Rich Cathode Materials via Heterostructured Spinel Li4Mn5O12 Coating. Adv. Mater. 2018, 30, 1801751. [Google Scholar] [CrossRef]
- Wang, N.; Chen, Y.; Yin, J.; Yan, W.; Li, F.; Jin, Y. Systematic Study of the Effects of Lithium Deficiencies on the Crystal Structure and Electrochemical Performance of Li-Rich Materials. J. Alloys Compd. 2022, 900, 163549. [Google Scholar] [CrossRef]
- Zhang, K.; Li, B.; Zuo, Y.; Song, J.; Shang, H.; Ning, F.; Xia, D. Voltage Decay in Layered Li-Rich Mn-Based Cathode Materials. Electrochem. Energy Rev. 2019, 2, 606–623. [Google Scholar] [CrossRef]
- Yan, P.; Nie, A.; Zheng, J.; Zhou, Y.; Lu, D.; Zhang, X.; Xu, R.; Belharouak, I.; Zu, X.; Xiao, J.; et al. Evolution of Lattice Structure and Chemical Composition of the Surface Reconstructions Layer in Li1.2Ni0.2Mn0.6O2 Cathode Material for Lithium Ion Batteries. Nano Lett. 2015, 15, 514–522. [Google Scholar] [CrossRef]
- Hua, W.; Wang, S.; Knapp, M.; Leake, S.J.; Senyshyn, A.; Richter, C.; Yavuz, M.; Binder, J.R.; Grey, C.P.; Ehrenberg, H.; et al. Structural Insights into the Formation and Voltage Degradation of Lithium- and Manganese-Rich Layered Oxides. Nat. Commun. 2019, 10, 5365. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Zhou, L.; Park, M.; Han, D.; Lee, G.-H.; Kang, S.; Lee, S.; Chen, M.; Hu, Z.; Zhang, K.; et al. Hysteresis Induced by Incomplete Cationic Redox in Li-Rich 3d-Transition-Metal Layered Oxides Cathodes. Adv. Sci. 2022, 9, 220196. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Shen, S.; Yan, X.; Wu, R.; Wu, A.; Lastoskie, C.; Zhang, J. Sacrificial Template Strategy toward a Hollow LiNi1/3Co1/3Mn1/3O2 Nanosphere Cathode for Advanced Lithium-Ion Batteries. ACS Omega 2017, 2, 7593–7599. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, M.; Yi, L.; Liang, K. Fe3+ and PO43- Co-Doped Li-Rich Li1.20Mn0.56Ni0.16Co0.08O2 as Cathode with Outstanding Structural Stability for Lithium-Ion Battery. J. Alloys Compd. 2021, 865, 158899. [Google Scholar] [CrossRef]
- An, J.; Shi, L.; Chen, G.; Li, M.; Liu, H.; Yuan, S.; Chen, S.; Zhang, D. Insights into the Stable Layered Structure of a Li-Rich Cathode Material for Lithium-Ion Batteries. J. Mater. Chem. A 2017, 5, 19738–19744. [Google Scholar] [CrossRef]
- Liang, Y.; Li, S.; Xie, J.; Yang, L.; Li, W.; Li, C.; Ai, L.; Fu, X.; Cui, X.; Shangguan, X. Synthesis and Electrochemical Characterization of Mg-Al Co-Doped Li-Rich Mn-Based Cathode Materials. New J. Chem. 2019, 43, 12004–12012. [Google Scholar] [CrossRef]
- Shanmugam, V.; Natarajan, S.; Lobo, L.S.; Mathur, A.; Sahana, M.B.; Sundararajan, G.; Gopalan, R. Surface Oxygen Vacancy Engineering and Physical Protection by In-Situ Carbon Coating Process of Lithium Rich Layered Oxide. J. Power Sources 2021, 515, 230623. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, J.; Wu, L.; Guo, Z.; Ji, Z.; Luo, J.; Chen, C.; Shu, J.; Long, H.; Yang, F.; et al. Enhanced Electrochemical Performance of Perovskite LaNiO3 Coating on Li1.2Mn0.54Ni0.13Co0.13O2 as Cathode Materials for Li-Ion Batteries. Electrochim. Acta 2018, 283, 1203–1212. [Google Scholar] [CrossRef]
- Chen, W.C.; Song, Y.F.; Wang, C.C.; Liu, Y.; Morris, D.T.; Pianetta, P.A.; Andrews, J.C.; Wu, H.C.; Wu, N.L. Study on the Synthesis-Microstructure-Performance Relationship of Layered Li-Excess Nickel-Manganese Oxide as a Li-Ion Battery Cathode Prepared by High-Temperature Calcination. J. Mater. Chem. A 2013, 1, 10847–10856. [Google Scholar] [CrossRef]
- Wang, M.-J.; Yu, F.-D.; Sun, G.; Gu, D.-M.; Wang, Z.-B. Optimizing the Structural Evolution of Li-Rich Oxide Cathode Materials via Microwave-Assisted Pre-Activation. ACS Appl. Energy Mater. 2018, 1, 4158–4168. [Google Scholar] [CrossRef]
- Bao, Y.; Wang, J.; Qian, Y.; Deng, Y.; Yang, X.; Chen, G. An Appropriate Amount of New Spinel Phase Induced by Control Synthesis for the Improvement of Electrochemical Performance of Li-Rich Layered Oxide Cathode Material. Electrochim. Acta 2020, 330, 135240. [Google Scholar] [CrossRef]






| Sample | c (Å) | a (Å) | c/a | I(003)/I(104) | (I(006)+I(012))/I(101) | Rwp (%) |
|---|---|---|---|---|---|---|
| w/o microwave | 14.2378 | 2.8513 | 4.9934 | 1.909 | 0.4431 | 13.16 |
| 10 min | 14.2418 | 2.8515 | 4.9945 | 1.923 | 0.4154 | 12.74 |
| 20 min | 14.2463 | 2.8521 | 4.9950 | 2.015 | 0.4011 | 12.98 |
| 30 min | 14.2492 | 2.8532 | 4.9940 | 1.946 | 0.4326 | 13.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karunawan, J.; Abdillah, O.B.; Floweri, O.; Aji, M.P.; Santosa, S.P.; Sumboja, A.; Iskandar, F. Improving the Structural Ordering and Particle-Size Homogeneity of Li-Rich Layered Li1.2Ni0.13Co0.13Mn0.54O2 Cathode Materials through Microwave Irradiation Solid-State Synthesis. Batteries 2023, 9, 31. https://doi.org/10.3390/batteries9010031
Karunawan J, Abdillah OB, Floweri O, Aji MP, Santosa SP, Sumboja A, Iskandar F. Improving the Structural Ordering and Particle-Size Homogeneity of Li-Rich Layered Li1.2Ni0.13Co0.13Mn0.54O2 Cathode Materials through Microwave Irradiation Solid-State Synthesis. Batteries. 2023; 9(1):31. https://doi.org/10.3390/batteries9010031
Chicago/Turabian StyleKarunawan, Jotti, Oktaviardi Bityasmawan Abdillah, Octia Floweri, Mahardika Prasetya Aji, Sigit Puji Santosa, Afriyanti Sumboja, and Ferry Iskandar. 2023. "Improving the Structural Ordering and Particle-Size Homogeneity of Li-Rich Layered Li1.2Ni0.13Co0.13Mn0.54O2 Cathode Materials through Microwave Irradiation Solid-State Synthesis" Batteries 9, no. 1: 31. https://doi.org/10.3390/batteries9010031