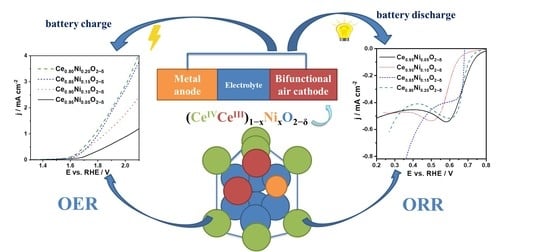
Nickel-Doped Ceria Bifunctional Electrocatalysts for Oxygen Reduction and Evolution in Alkaline Media
Abstract
:1. Introduction
2. Results
2.1. General Properties of the Ce1−xNixO2−δ Powders
2.2. Capacitance Study
2.3. ORR Studies
2.4. OER Studies
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ralph, T.R.; Hogarth, M.P. Catalysis for low temperature fuel cells. Platin. Met. Rev. 2002, 46, 117–135. [Google Scholar] [CrossRef]
- Mota, N.D.; Finkelstein, I.D.; Kirtland, I.J.D.; Claudia, I.; Stroock, A.D.; Abruña, H.D. Membraneless, Room-Temperature, Direct Borohydride/Cerium Fuel Cell with Power Density of Over 0.25 W cm−2. J. Am. Chem. Soc. 2012, 134, 6076–6079. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Xia, Y.Y. A direct borohydride fuel cell using MnO2-catalyzed cathode and hydrogen storage alloy anode. Electrochem. Commun. 2006, 8, 1775–1778. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Q.; Wu, C.; Wang, Y.; Wei, W.; Wang, X.; Yi, L. Performance improvement of activated nanoporous carbon supported gold catalyst as an anode for direct borohydride–hydrogen peroxide fuel cells. RSC Adv. 2014, 4, 17129–17135. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Y.; Zhang, P.; Wang, J. A simple direct borohydride fuel cell with a cobalt phthalocyanine catalyzed cathode. Electrochem. Commun. 2008, 10, 100–102. [Google Scholar] [CrossRef]
- Šljukić, B.; Santos, D.M.F.; Sequeira, C.A.C. Manganese dioxide electrocatalysts for borohydride fuel cell cathodes? J. Electroanal. Chem. 2013, 694, 77–83. [Google Scholar] [CrossRef]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: A comparative study of nanoparticles and bulk materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Mosiałek, M.; Nowak, P.; Dudek, M.; Mordarski, G. Oxygen reduction at the Ag|Gd0.2Ce0.8O1.9 interface studied by electrochemical impedance spectroscopy and cyclic voltammetry at the silver point electrode. Electrochim. Acta 2014, 120, 248–257. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Fan, L.; Han, J.; Xiong, Y. Fe/Ni-N-CNFs electrochemical catalyst for oxygen reduction reaction/oxygen evolution reaction in alkaline media. Appl. Surf. Sci. 2017, 401, 89–99. [Google Scholar] [CrossRef]
- Lee, D.U.; Kim, B.J.; Chen, Z. One-pot synthesis of a mesoporous NiCo2O4 nanoplatelet and graphene hybrid and its oxygen reduction and evolution activities as an efficient bi-functional electrocatalyst. J. Mater. Chem. A 2013, 1, 4754. [Google Scholar] [CrossRef]
- Choi, B.; Lee, S.; Fushimi, C.; Tsutsumi, A. Development of NiMH-based Fuel Cell/Battery (FCB) system: Characterization of Ni(OH)2/MnO2 positive electrode for FCB. J. Power Sources 2009, 194, 1150–1155. [Google Scholar] [CrossRef]
- Dar, Y.R.; Vijay, P.; Tade, M.O.; Datta, R. Topological analysis of hydrogen oxidation reaction kinetics at Ni/YSZ anode of the solid oxide fuel cell. J. Electroanal. Chem. 2012, 677–680, 15–23. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, A.; Higgins, D.; Li, H.; Wang, H.; Chen, Z. Highly active and durable core-corona structured bifunctional catalyst for rechargeable metal-air battery application. Nano Lett. 2012, 12, 1946–1952. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.T.; Cao, R.; Choi, N.S.; Liu, M.; Lee, K.T.; Cho, J. Metal-air batteries with high energy density: Li-air versus Zn-air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.; Xia, Z.; Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 2015, 10, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Bing, Y.; Liu, H.; Zhang, L.; Ghosh, D.; Zhang, J. Nanostructured Pt-alloy electrocatalysts for PEM fuel cell oxygen reduction reaction. Chem. Soc. Rev. 2010, 39, 2184. [Google Scholar] [CrossRef]
- Higgins, D.C.; Meza, D.; Chen, Z. Nitrogen-Doped Carbon Nanotubes as Platinum Catalyst Supports for Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cells. J. Phys. Chem. C 2010, 114, 21982–21988. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B Environ. 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Schulze, M.; Wagner, N.; Kaz, T.; Friedrich, K.A. Combined electrochemical and surface analysis investigation of degradation processes in polymer electrolyte membrane fuel cells. Electrochim. Acta 2007, 52, 2328–2336. [Google Scholar] [CrossRef]
- Sheng, X.; Daems, N.; Geboes, B.; Kurttepeli, M.; Bals, S.; Breugelmans, T.; Hubin, A.; Vankelecom, I.F.J.; Pescarmona, P.P. N-doped ordered mesoporous carbons prepared by a two-step nanocasting strategy as highly active and selective electrocatalysts for the reduction of O2 to H2O2. Appl. Catal. B Environ. 2015, 176–177, 212–224. [Google Scholar] [CrossRef]
- Meng, Y.; Song, W.; Huang, H.; Ren, Z.; Chen, S.; Suib, S.L. Structure-Property Relationship of Bifunctional MnO2 Nanostructures: Highly Efficient, Ultra-Stable Electrochemical Water Oxidation and Oxygen Reduction Reaction Catalysts Identified in Alkaline Media. J. Am. Chem. Soc. 2014, 136, 11452–11464. [Google Scholar] [CrossRef] [PubMed]
- Tseung, A.C.C.; Jasem, S. Oxygen evolution on semiconducting oxides. Electrochim. Acta 1977, 22, 31–34. [Google Scholar] [CrossRef]
- Lyons, M.E.G.; Brandon, M.P. A comparative study of the oxygen evolution reaction on oxidised nickel, cobalt and iron electrodes in base. J. Electroanal. Chem. 2010, 641, 119–130. [Google Scholar] [CrossRef]
- Jin, H.; Yu, H.; Li, H.; Davey, K.; Song, T.; Paik, U.; Qiao, S.-Z. MXene Analogue: A 2D Nitridene Solid Solution for High-Rate Hydrogen Production. Angew. Chem. Int. Ed. 2022, 61, e202203850. [Google Scholar] [CrossRef]
- Jin, H.; Song, T.; Paik, U.; Qiao, S.-Z. Metastable Two-Dimensional Materials for Electrocatalytic Energy Conversions. Acc. Mater. Res. 2021, 2, 559–573. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Gu, J.; Su, L.; Cheng, L. An overview of metal oxide materials as electrocatalysts and supports for polymer electrolyte fuel cells. Energy Environ. Sci. 2014, 7, 2535–2558. [Google Scholar] [CrossRef]
- Deng, J.; Chu, W.; Wang, B.; Yang, W.; Zhao, X.S. Mesoporous Ni/Ce1-xNixO2-y heterostructure as an efficient catalyst for converting greenhouse gas to H2 and syngas. Catal. Sci. Technol. 2016, 6, 851–862. [Google Scholar] [CrossRef]
- Shan, W.; Luo, M.; Ying, P.; Shen, W.; Li, C. Reduction property and catalytic activity of Ce1-XNiXO2 mixed oxide catalysts for CH4 oxidation. Appl. Catal. A Gen. 2003, 246, 1–9. [Google Scholar] [CrossRef]
- Zhou, G.; Barrio, L.; Agnoli, S.; Senanayake, S.D.; Evans, J.; Kubacka, A.; Estrella, M.; Hanson, J.C.; Martínez-Arias, A.; Fernández-García, M.; et al. High activity of Ce1-xNixO2-y for H2 production through ethanol steam reforming: Tuning catalytic performance through metal-oxide interactions. Angew. Chem. Int. Ed. 2010, 49, 9680–9684. [Google Scholar] [CrossRef] [PubMed]
- Elias, J.S.; Risch, M.; Giordano, L.; Mansour, A.N.; Shao-Horn, Y. Structure, bonding, and catalytic activity of monodisperse, transition-metal-substituted CeO2 nanoparticles. J. Am. Chem. Soc. 2014, 136, 17193–17200. [Google Scholar] [CrossRef]
- Barrio, L.; Kubacka, A.; Zhou, G.; Estrella, M.; Martínez-Arias, A.; Hanson, J.C.; Fernández-García, M.; Rodriguez, J.A. Unusual Physical and Chemical Properties of Ni in Ce1−xNixO2−y Oxides: Structural Characterization and Catalytic Activity for the Water Gas Shift Reaction. J. Phys. Chem. C 2010, 114, 12689–12697. [Google Scholar] [CrossRef]
- Tao, Z.; Hou, G.; Xu, N.; Zhang, Q. A highly coking-resistant solid oxide fuel cell with a nickel doped ceria: Ce1-xNixO2-y reformation layer. Int. J. Hydrogen Energy 2014, 39, 5113–5120. [Google Scholar] [CrossRef]
- Tan, Q.; Du, C.; Sun, Y.; Du, L.; Yin, G.; Gao, Y. Nickel-doped ceria nanoparticles for promoting catalytic activity of Pt/C for ethanol electrooxidation. J. Power Sources 2014, 263, 310–314. [Google Scholar] [CrossRef]
- Parwaiz, S.; Bhunia, K.; Das, A.K.; Khan, M.M.; Pradhan, D. Cobalt-Doped Ceria/Reduced Graphene Oxide Nanocomposite as an Efficient Oxygen Reduction Reaction Catalyst and Supercapacitor Material. J. Phys. Chem. C 2017, 121, 20165–20176. [Google Scholar] [CrossRef]
- Yang, Z.-B.; Yue, T.-L.; Yu, X.-N.; Wu, M.-M. Electrocatalytic Activity of Cobalt Doped Ceria Nanoparticles. J. Inorg. Mater. 2018, 33, 845–853. [Google Scholar]
- Meléndez-González, P.C.; Sánchez-Castro, E.; Alonso-Lemus, I.L.; Pérez-Hernández, R.; Escobar-Morales, B.; Garay-Tapia, A.M.; Pech-Rodríguez, W.J.; Rodríguez-Varela, J. Bifunctional Pd-CeO2 Nanorods/C Nanocatalyst with High Electrochemical Stability and Catalytic Activity for the ORR and EOR in Alkaline Media. ChemistrySelect 2020, 5, 14032–14040. [Google Scholar] [CrossRef]
- Sridharan, M.; Maiyalagan, T. Enhanced oxygen reduction activity of bimetallic Pd-Ag alloy-supported on mesoporous cerium oxide electrocatalysts in alkaline media. New J. Chem. 2021, 45, 22181–22192. [Google Scholar] [CrossRef]
- Xu, S.; Lv, C.; He, T.; Huang, Z.; Zhang, C. Amorphous film of cerium doped cobalt oxide as a highly efficient electrocatalyst for oxygen evolution reaction. J. Mater. Chem. A 2019, 7, 7526–7532. [Google Scholar] [CrossRef]
- Bhuvanendran, N.; Ravichandran, S.; Kandasamy, S.; Zhang, W.; Xu, Q.; Khotseng, L.; Maiyalagan, T.; Su, H. Spindle-shaped CeO2/biochar carbon with oxygen-vacancy as an effective and highly durable electrocatalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 2021, 46, 2128–2142. [Google Scholar] [CrossRef]
- Yu, H.; Davydova, E.S.; Ash, U.; Miller, H.A.; Bonville, L.; Dekel, D.R.; Maric, R. Palladium-ceria nanocatalyst for hydrogen oxidation in alkaline media: Optimization of the Pd–CeO2 interface. Nano Energy 2019, 57, 820–826. [Google Scholar] [CrossRef]
- Kuzmanović, B.; Vujković, M.J.; Tomić, N.; Bajuk-Bogdanović, D.; Lazović, V.; Šljukić, B.; Ivanović, N.; Mentus, S. The influence of oxygen vacancy concentration in nanodispersed non-stoichiometric CeO2-δ oxides on the physico-chemical properties of conducting polyaniline/CeO2 composites. Electrochim. Acta 2019, 306, 506–515. [Google Scholar] [CrossRef]
- Fuentes, R.O.; Acuña, L.M.; Albornoz, C.A.; Leyva, A.G.; Sousa, N.; Figueiredo, F.M. Structural, physical and chemical properties of nanostructured nickel-substituted ceria oxides under reducing and oxidizing conditions. RSC Adv. 2016, 6, 64861–64870. [Google Scholar] [CrossRef]
- Shannon, R.D. Application of the Periodic Bond Chain (PBC) Theory and Attachment Energy Consideration to Derive the Crystal Morphology of Hexamethylmelamine. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Mentus, S.; Vujković, M.; Gavrilov, N.; Pašti, I.; Krstić, J.; Travas-Sejdic, J.; Ćirić-Marjanović, G. Superior capacitive and electrocatalytic properties of carbonized nanostructured polyaniline upon a low-temperature hydrothermal treatment. Carbon 2013, 64, 472–486. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, X.; Pan, Y.; Peng, Z. A review of Pt-based electrocatalysts for oxygen reduction reaction. Front. Energy 2017, 11, 268–285. [Google Scholar] [CrossRef]
- Qiu, K.; Chai, G.; Jiang, C.; Ling, M.; Tang, J.; Guo, Z. Highly Efficient Oxygen Reduction Catalysts by Rational Synthesis of Nanoconfined Maghemite in a Nitrogen-Doped Graphene Framework. ACS Catal. 2016, 6, 3558–3568. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Ponraj, J.; Mansour, S.A.; Tarlochan, F. Highly active and stable bi-functional NiCoO2 catalyst for oxygen reduction and oxygen evolution reactions in alkaline medium. Int. J. Hydrogen Energy 2019, 44, 16603–16614. [Google Scholar] [CrossRef]
- Sandhiran, N.; Ganapathy, S.; Manoharan, Y.; Ganguly, D.; Kumar, M.; Ramanujam, K.; Balachandran, S. CuO–NiO binary transition metal oxide nanoparticle anchored on rGO nanosheets as high-performance electrocatalyst for the oxygen reduction reaction. Environ. Res. 2022, 211, 112992. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, N.; Liang, Y. Preparation of CeO2/Cu-MOF/GO composite for efficient electrocatalytic oxygen evolution reaction. Ionics 2021, 27, 4347–4360. [Google Scholar] [CrossRef]
- Plevová, M.; Hnát, J.; Bouzek, K. Electrocatalysts for the oxygen evolution reaction in alkaline and neutral media. A comparative review. J. Power Sources 2021, 507. [Google Scholar] [CrossRef]
- Milikić, J.; Ćirić-Marjanović, G.; Mentus, S.; Santos, D.M.F.; Sequeira, C.A.C.; Šljukić, B. Pd/c-PANI electrocatalysts for direct borohydride fuel cells. Electrochim. Acta 2016, 213, 298–305. [Google Scholar] [CrossRef]
- Liu, D.M.; Huang, W.J.; Si, T.Z.; Zhang, Q.A. Hydrogen storage properties of LiBH4 destabilized by SrH2. J. Alloy. Compd. 2013, 551, 8–11. [Google Scholar] [CrossRef]
- Suffredini, H.B.; Cerne, J.L.; Crnkovic, F.C.; Machado, S.A.S.; Avaca, L.A. Recent developments in electrode materials for water electrolysis. Int. J. Hydrogen Energy 2000, 25, 415–423. [Google Scholar] [CrossRef]
- Lu, B.; Cao, D.; Wang, P.; Wang, G.; Gao, Y. Oxygen evolution reaction on Ni-substituted Co3O4 nanowire array electrodes. Int. J. Hydrogen Energy 2011, 36, 72–78. [Google Scholar] [CrossRef]




| x in Ce1−xNixO2−δ | a (Å) | %(Ce,Ni)O2: %NiO | DXRD (nm) | SBET (m2 g−1) | DBET (nm) | DBET: DXRD | Pore Volume (cm3 g−1) |
|---|---|---|---|---|---|---|---|
| 0 | 5.4144(6) | − | 11.9 | 26 | 35 | 2.9 | − |
| 0.05 | 5.4150(6) | 100:0 | 7.1 | 39 | 22 | 3.1 | 0.070 |
| 0.10 | 5.4149(6) | 100:0 | 5.9 | 37 | 24 | 4.1 | 0.048 |
| 0.15 | 5.4163(3) | 98:2 | 5.6 | 31 | 29 | 5.2 | 0.040 |
| 0.20 | 5.4173(2) | 96:4 | 5.0 | 35 | 26 | 5.2 | 0.044 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milikić, J.; Fuentes, R.O.; Tasca, J.E.; Santos, D.M.F.; Šljukić, B.; Figueiredo, F.M.L. Nickel-Doped Ceria Bifunctional Electrocatalysts for Oxygen Reduction and Evolution in Alkaline Media. Batteries 2022, 8, 100. https://doi.org/10.3390/batteries8080100
Milikić J, Fuentes RO, Tasca JE, Santos DMF, Šljukić B, Figueiredo FML. Nickel-Doped Ceria Bifunctional Electrocatalysts for Oxygen Reduction and Evolution in Alkaline Media. Batteries. 2022; 8(8):100. https://doi.org/10.3390/batteries8080100
Chicago/Turabian StyleMilikić, Jadranka, Rodolfo O. Fuentes, Julia E. Tasca, Diogo M. F. Santos, Biljana Šljukić, and Filipe M. L. Figueiredo. 2022. "Nickel-Doped Ceria Bifunctional Electrocatalysts for Oxygen Reduction and Evolution in Alkaline Media" Batteries 8, no. 8: 100. https://doi.org/10.3390/batteries8080100