Advances in Battery Materials
Share This Topical Collection
Editor
 Prof. Dr. Seung-Tae Hong
Prof. Dr. Seung-Tae Hong
 Prof. Dr. Seung-Tae Hong
Prof. Dr. Seung-Tae Hong
E-Mail
Website
Collection Editor
Department of Energy Science and Engineering, DGIST (Daegu Gyeongbuk Institute of Science and Technology), Daegu 42988, Republic of Korea
Interests: all-solid-state batteries (Li, Na); Mg-ion batteries; Ca-ion batteries; Li-ion batteries; X-ray crystallography
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Rechargeable batteries have become essential components in our daily lives. During recent decades, their applications have been rapidly expanded from portable electronic devices to electric vehicles, drones, large-scale energy storage systems, and electric aircrafts, which are under development. In particular, the relatively new and emerging applications require much higher performance standards—in terms of energy density, power, cycle life, and safety—than that which commercialized state-of-the-art lithium-ion batteries (LIBs) can provide. The materials comprising the batteries inherently determine performances, primarily the cathodes, anodes, electrolytes, and separators. During the last three decades, vigorous developments of battery materials have significantly improved battery performances, and further developments are under way.
On the other hand, during the last decade, post-lithium-ion batteries have received considerable attention due to safety concerns of LIBs, uneven distribution of lithium resources on the Earth’s crust, and the demand for higher energy density. Intense research activities are underway to develop all-solid-state batteries, Li-S batteries, and non-lithium-based batteries, including zinc-, sodium-, potassium-, magnesium-, and calcium-ion batteries.
For this Topical Collection of Batteries, we warmly welcome the submission of original research articles or reviews on topics related to advances in battery materials, including synthesis, processes, physicochemical characterization, computational analysis, and mechanism analysis. Topics of interest for publication include, but are not limited to, the following:
- Synthesis and processing studies on cathode and anode materials for LIBs, Li-S, Na-ion, K-ion, Zn-ion, Mg-ion, and Ca-ion batteries;
- Structural and morphological analysis of electrode materials;
- Electrochemical performance studies of electrode materials;
- Electrochemical reaction mechanism studies;
- Spectroscopic studies on electrode materials;
- Theoretical and computational studies on electrode materials;
- Physicochemical characterizations of electrode materials.
Prof. Dr. Seung-Tae Hong
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Batteries is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- lithium-ion batteries
- sodium-ion batteries
- potassium-ion batteries
- Li-S batteries
- zinc-ion batteries
- magnesium-ion batteries
- calcium-ion batteries
- all-solid-state batteries
- post-lithium-ion battery materials
Published Papers (18 papers)
Open AccessReview
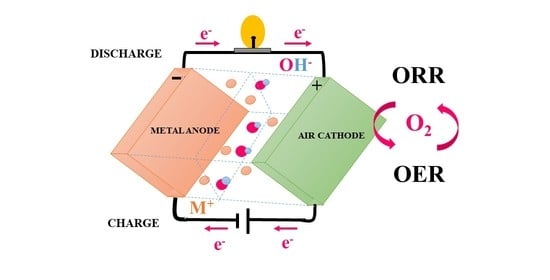
Air Cathodes and Bifunctional Oxygen Electrocatalysts for Aqueous Metal–Air Batteries
by
Jadranka Milikić, Ana Nastasić, Marta Martins, César A. C. Sequeira and Biljana Šljukić
Cited by 4 | Viewed by 1645
Abstract
One of the most popular solutions for electrochemical energy storage is metal–air batteries, which could be employed in electric vehicles or grid energy storage. Metal–air batteries have a higher theoretical energy density than lithium-ion batteries. The crucial components for the best performance of
[...] Read more.
One of the most popular solutions for electrochemical energy storage is metal–air batteries, which could be employed in electric vehicles or grid energy storage. Metal–air batteries have a higher theoretical energy density than lithium-ion batteries. The crucial components for the best performance of batteries are the air cathode electrocatalysts and corresponding electrolytes. Herein, we present several of the latest studies on electrocatalysts for air cathodes and bifunctional oxygen electrocatalysts for aqueous zinc–air and aluminium–air batteries.
Full article
►▼
Show Figures
Open AccessArticle
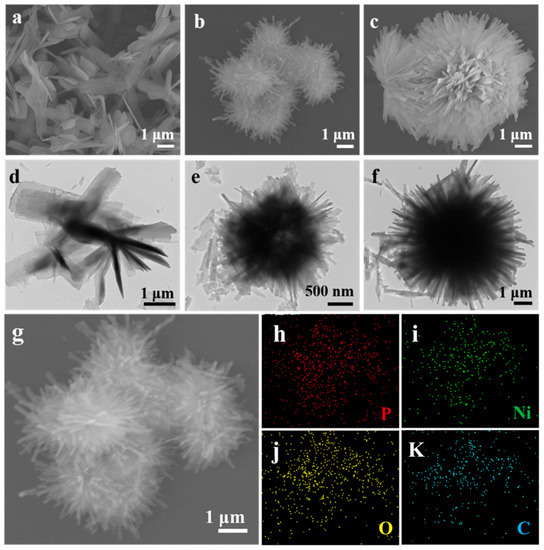
A Nickel-Based Coordination Compound with Tunable Morphology for High-Performance Anode and the Lithium Storage Mechanism
by
Yifei Lu, Lei Wang, Zhenzhu Lou, Leilei Wang, Yi Zhao, Weiwei Sun, Liping Lv, Yong Wang and Shuangqiang Chen
Viewed by 1273
Abstract
Metal-organic coordination compounds (MCCs) have received a lot of attention as anodes for lithium-ion batteries (LIBs) due to their abundant structural configuration, tunable morphology, high surface area, and low cost, but the lithium storage mechanism of MCCs is still a mystery. Herein, we
[...] Read more.
Metal-organic coordination compounds (MCCs) have received a lot of attention as anodes for lithium-ion batteries (LIBs) due to their abundant structural configuration, tunable morphology, high surface area, and low cost, but the lithium storage mechanism of MCCs is still a mystery. Herein, we synthesized a kind of nickel-based coordination compound (marked as Ni-PP-x, x = 1, 2, or 3) with tunable morphologies and different solvent ratios via a microwave irradiation solvothermal method and then applied them as anodes for LIBs. Among them, the Ni-PP-2 electrode, with a hollow and urchin-like structure, showed the longest lifespan and maintained a high capacity of 713 mAh g
−1 at 2.0 A g
−1 after 800 cycles. Measured by ex situ X-ray photoelectron spectroscopy (XPS) and ex situ Fourier transform infrared spectroscopy (FT-IR), the Ni-PP-2 electrode was confirmed by a redox reaction mechanism of Li
+ cations with a benzene ring and O-Ni
2+/O-Ni
0 coordination bonds, and the cyclic voltammetry curves have exhibited a capacitive dominated lithium storage behavior. This work provides a new type of Ni-based coordination compound and an in-depth understanding of their lithium storage mechanism, paving the way for the application of MCC compounds in the future.
Full article
►▼
Show Figures
Open AccessArticle
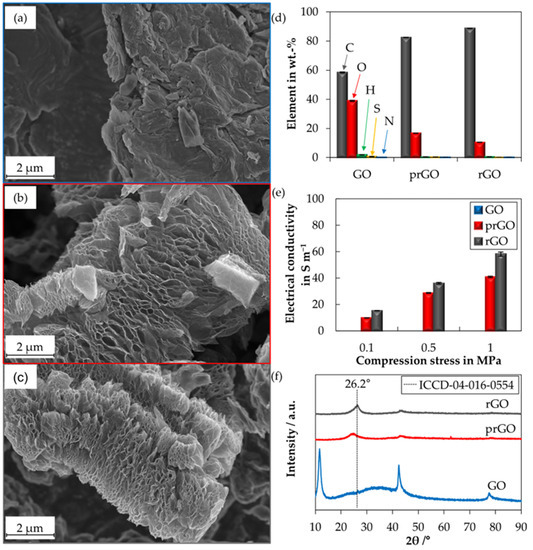
Effect of Water-Soluble CMC/SBR Binder Ratios on Si-rGO Composites Using µm- and nm-Sized Silicon as Anode Materials for Lithium-Ion Batteries
by
Sebastian Müllner, Tobias Michlik, Michael Reichel, Tilo Held, Ralf Moos and Christina Roth
Cited by 5 | Viewed by 3462
Abstract
Silicon-containing materials are still the most promising alternatives to graphite as the negative electrodes of lithium-ion batteries. However, the different Li
+ storage mechanism combined with the high capacity result in new requirements for the passive electrode components, such as the binder. To
[...] Read more.
Silicon-containing materials are still the most promising alternatives to graphite as the negative electrodes of lithium-ion batteries. However, the different Li
+ storage mechanism combined with the high capacity result in new requirements for the passive electrode components, such as the binder. To ensure sufficient cycling stability, silicon must be embedded in a suitable carbonaceous matrix. For this purpose, we used a simple ball milling process with reduced graphene oxide (rGO) to produce Si-rGO composites with µm- and nm-sized silicon particles. The rGO was synthesized previously from a two-step thermal synthesis method developed in-house. Subsequently, electrodes with varying CMC/SBR ratios (3:1, 1:1, and 1:3) were prepared from the composites containing the different Si particle sizes. It was found that the optimal binder ratio depends on the size of the Si particles. For the nm-Si-rGO composite, a CMC/SBR ratio of 3:1 results in a total capacity over 51 cycles of 20.6 Ah g
−1, which means an improvement of 20% compared to CMC/SBR = 1:3 (17.1 Ah g
−1). In contrast, we demonstrate that for µm-Si-rGO composites with an optimal CMC/SBR ratio of 1:1 (13.0 Ah g
−1), compared to nm-Si-rGO, a higher SBR content is beneficial for the cycling behavior. Moreover, a comparison with graphite from the literature indicates that a rGO-matrix reduces the need for SBR.
Full article
►▼
Show Figures
Open AccessArticle
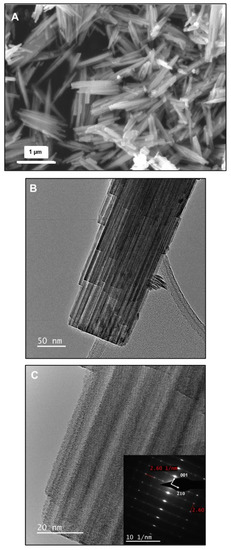
Charge Storage Mechanism of LixWO3 Hexagonal Tungsten Bronze in Aqueous Electrolytes
by
Julio César Espinosa-Angeles, Eric Quarez, Louis-Béni Mvele Eyé’a, Camille Douard, Antonella Iadecola, Hui Shao, Pierre-Louis Taberna, Patrice Simon, Olivier Crosnier and Thierry Brousse
Cited by 1 | Viewed by 1832
Abstract
The electrochemical behavior of the lithium hexagonal tungsten bronze, Li
xWO
3, is investigated herein. The material was synthesized at a low temperature under hydrothermal conditions, yielding nanorod-like particles with growth along the c-axis. Upon cycling in a 5 M LiNO
[...] Read more.
The electrochemical behavior of the lithium hexagonal tungsten bronze, Li
xWO
3, is investigated herein. The material was synthesized at a low temperature under hydrothermal conditions, yielding nanorod-like particles with growth along the c-axis. Upon cycling in a 5 M LiNO
3 aqueous electrolyte, a specific capacity of 71 C.g
−1 was obtained at 2 mV.s
−1, corresponding to a charge/discharge cycle of 10 min. The charge storage mechanism was elucidated using various complementary techniques, such as electrochemical quartz crystal microbalance (EQCM) and synchrotron
operando X-ray absorption spectroscopy (XAS). A desolvation process upon Li
+ intercalation into the lattice of the material was evidenced, accompanied by a reversible reduction/oxidation of tungsten cations in the crystal structure upon charge/discharge cycling.
Full article
►▼
Show Figures
Open AccessArticle
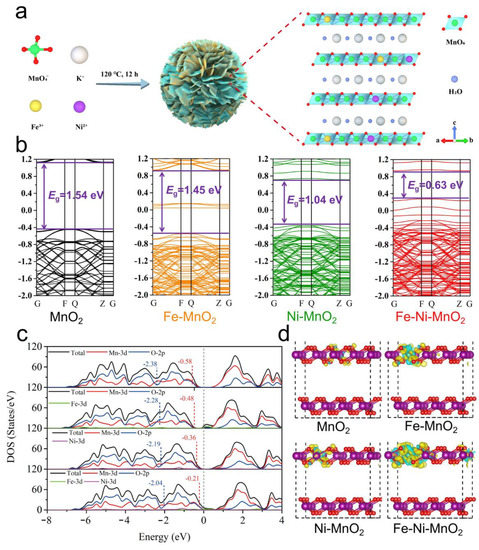
Ni/Fe Bimetallic Ions Co-Doped Manganese Dioxide Cathode Materials for Aqueous Zinc-Ion Batteries
by
Feifei Gao, Wenchao Shi, Bowen Jiang, Zhenzhi Xia, Lei Zhang and Qinyou An
Viewed by 2726
Abstract
The slow diffusion dynamics hinder aqueous MnO
2/Zn batteries’ further development. Here, a Ni/Fe bimetallic co-doped MnO
2 (NFMO) cathode material was studied by density functional theory (DFT) calculation and experimental characterization techniques, such as cyclic voltammetry (CV), galvanostatic intermittent titration technique
[...] Read more.
The slow diffusion dynamics hinder aqueous MnO
2/Zn batteries’ further development. Here, a Ni/Fe bimetallic co-doped MnO
2 (NFMO) cathode material was studied by density functional theory (DFT) calculation and experimental characterization techniques, such as cyclic voltammetry (CV), galvanostatic intermittent titration technique (GITT) and electrochemical impedance spectra (EIS). The results indicated that the energy band structure and electronic state of MnO
2 were effectively optimized due to the simultaneous incorporation of strongly electronegative Ni and Fe ions. Consequently, the NFMO cathode material exhibited a faster charge transfer and ion diffusion dynamics than MnO
2 (MO), thus, the assembled NFMO/Zn batteries delivered excellent rate performance (181 mA h g
−1 at 3 A g
−1). The bimetallic ions co-doping strategy provides new directions for the development of oxide cathode materials towards high-performance aqueous zinc-ion batteries.
Full article
►▼
Show Figures
Open AccessFeature PaperEditor’s ChoiceArticle
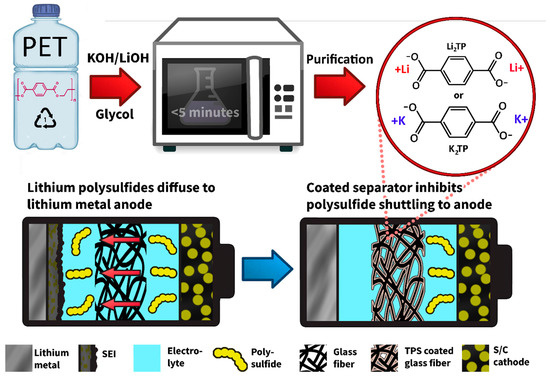
Mitigating Polysulfide Shuttles with Upcycled Alkali Metal Terephthalate Decorated Separators
by
Daniel A. Gribble, Zih-Yu Lin, Sourav Ghosh, Brett M. Savoie and Vilas G. Pol
Cited by 1 | Viewed by 2521
Abstract
High energy density lithium–sulfur batteries (LSBs) are a potential replacement for lithium-ion batteries (LIBs). However, practical lifetimes are inhibited by lithium polysulfide (LiPS) shuttling. Concurrently, plastic waste accumulation worldwide threatens our ecosystems. Herein, a fast and facile strategy to upcycle polyethylene terephthalate (PET)
[...] Read more.
High energy density lithium–sulfur batteries (LSBs) are a potential replacement for lithium-ion batteries (LIBs). However, practical lifetimes are inhibited by lithium polysulfide (LiPS) shuttling. Concurrently, plastic waste accumulation worldwide threatens our ecosystems. Herein, a fast and facile strategy to upcycle polyethylene terephthalate (PET) waste into useful materials is investigated. Dilithium terephthalate (Li
2TP) and dipotassium terephthalate (K
2TP) salts were synthesized from waste soda bottles via microwave depolymerization and solution coated onto glass fiber paper (GFP) separators. Salt-functionalized separators with Li
2TP@GFP and K
2TP@GFP mitigated LiPS shuttling and improved electrochemical performance in cells. Pore analysis and density functional theory (DFT) calculations indicate the action mechanism is synergistic physical blocking of bulky LiPS anions in nanopores and diffusion inhibition via electrostatic interactions with abundant carboxylate groups. LSBs with K
2TP@GFP separator showing highest LiPS affinity and smallest pore size demonstrated enhanced initial capacity as compared to non-modified GFP by 5.4% to 648 mAh g
−1, and increased cycle 100 capacity by 23% to 551 mAh g
−1. Overall, K
2TP@GFP retained 85% of initial capacity after 100 cycles with an average capacity fading of 0.15% per cycle. By comparison, GFP retained only 73% of initial capacity after 100 cycles with 0.27% average capacity loss, demonstrating effective LiPS retention.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
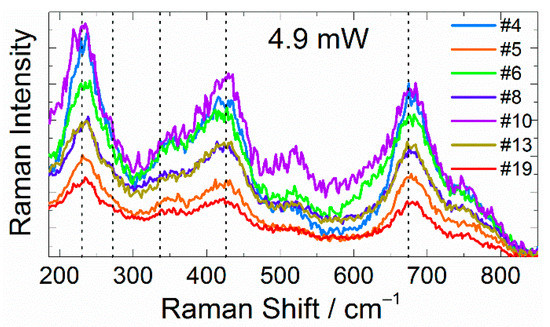
Fast and Slow Laser-Stimulated Degradation of Mn-Doped Li4Ti5O12
by
Aleksey A. Nikiforov, Dmitrii K. Kuznetsov, Ralph N. Nasara, Kaviarasan Govindarajan, Shih-kang Lin and Dmitry V. Pelegov
Cited by 2 | Viewed by 1296
Abstract
Lithium titanate (Li
4Ti
5O
12) is a commercial anode material used for high-power and long-lifespan lithium batteries. The key drawback of this material is its low electronic conductivity. Although doping is commonly used to solve this problem, the introduction
[...] Read more.
Lithium titanate (Li
4Ti
5O
12) is a commercial anode material used for high-power and long-lifespan lithium batteries. The key drawback of this material is its low electronic conductivity. Although doping is commonly used to solve this problem, the introduction of dopants also diminished lattice stability. In this work, we studied fast and slow laser-induced degradation processes of single Mn-doped lithium titanate particles and proposed a physicochemical model of their degradation mechanism. We suppose that the preferable route of LTO alteration is the formation of amorphous phases rather than crystalline decomposition products. Our results may be useful for not only developing a nondestructive characterization tool utilizing Raman spectroscopy but also for understanding other degradation processes, including thermal alteration and structural changes caused by the intercalation/deintercalation cycles of lithium ions.
Full article
►▼
Show Figures
Open AccessEditor’s ChoiceArticle
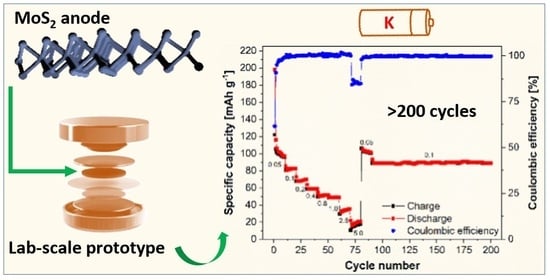
An Exploratory Study of MoS2 as Anode Material for Potassium Batteries
by
Lucia Fagiolari, Daniele Versaci, Federica Di Berardino, Julia Amici, Carlotta Francia, Silvia Bodoardo and Federico Bella
Cited by 15 | Viewed by 2580
Abstract
Potassium-based batteries represent one of the emerging classes of post-lithium electrochemical energy storage systems in the international scene, due to both the abundance of raw materials and achievable cell potentials not far from those of lithium batteries. In this context, it is important
[...] Read more.
Potassium-based batteries represent one of the emerging classes of post-lithium electrochemical energy storage systems in the international scene, due to both the abundance of raw materials and achievable cell potentials not far from those of lithium batteries. In this context, it is important to define electrodes and electrolytes that give reproducible performance and that can be used by different research groups as an internal standard when developing new materials. We propose molybdenum disulfide (MoS
2) as a valid anode choice, being a commercial and easily processable material, the 2D layered structure of which is promising for large potassium ions reversible storage. It has been proven to work for hundreds of cycles, keeping a constant specific capacity around 100 mAh g
−1 while also preserving its electrochemical interphase and morphology.
Full article
►▼
Show Figures
Open AccessArticle
Electrospun Interconnected Bead-Like P2-NaxCoyMn1−yO2 (x = 0.66, y = 0.1) Cathode Material for Stable Sodium-Ion Storage
by
Anupriya K. Haridas, Milan K. Sadan, Joo-Hyung Kim, Younki Lee and Jou-Hyeon Ahn
Cited by 3 | Viewed by 2058
Abstract
The development of high-rate and long-cycle-life Na-based cathode materials, on par with the performance of commercialized lithium-based cathodes, is crucial to satisfy the recurring surge in energy demand. Here, we report an interconnected bead-like P2-type manganese-based oxide Na
xCo
yMn
1−y
[...] Read more.
The development of high-rate and long-cycle-life Na-based cathode materials, on par with the performance of commercialized lithium-based cathodes, is crucial to satisfy the recurring surge in energy demand. Here, we report an interconnected bead-like P2-type manganese-based oxide Na
xCo
yMn
1−yO
2 (x = 0.66, y = 0.1) synthesized by electrospinning and subsequent heat treatment as a high-rate cathode material for sodium-ion batteries (SIBs). The employed strategy of one-dimensional morphological design with interconnected bead-like particles profusely enhances Na
+ diffusion pathways. This layered cathode material exhibits a stable and superior discharge capacity of 180.0 mAh g
−1 at 50 mA g
−1 compared to a bare cathode material synthesized via the sol–gel process. Further, a high capacity of 78.3 mAh g
−1 was achieved, maintaining excellent capacity retention of 85.0% even after 500 insertion/desertion cycles implying robust Na
+ storage properties. High-rate tests also revealed promising electrochemical performances at C-rates as high as 5000 mA g
−1, affirming the potential of this layered cathode material for high-rate Na
+ storage. Additionally, full SIBs assembled with a Na
xCo
yMn
1−yO
2 (x = 0.66, y = 0.1) cathode and a carbon nanofiber (CNF) anode exhibited a high cycle performance, retaining 96.3 mAh g
−1 after 100 cycles at 300 mA g
−1.
Full article
►▼
Show Figures
Open AccessEditor’s ChoiceArticle
Deep Learning Classification of Li-Ion Battery Materials Targeting Accurate Composition Classification from Laser-Induced Breakdown Spectroscopy High-Speed Analyses
by
Marie-Chloé Michaud Paradis, François R. Doucet, Steeve Rousselot, Alex Hernández-García, Kheireddine Rifai, Ouardia Touag, Lütfü Ç. Özcan, Nawfal Azami and Mickaël Dollé
Cited by 3 | Viewed by 2586
Abstract
Laser-induced breakdown spectroscopy (LIBS) is a valuable tool for the solid-state elemental analysis of battery materials. Key advantages include a high sensitivity for light elements (lithium included), complex emission patterns unique to individual elements through the full periodic table, and record speed analysis
[...] Read more.
Laser-induced breakdown spectroscopy (LIBS) is a valuable tool for the solid-state elemental analysis of battery materials. Key advantages include a high sensitivity for light elements (lithium included), complex emission patterns unique to individual elements through the full periodic table, and record speed analysis reaching 1300 full spectra per second (1.3 kHz acquisition rate). This study investigates deep learning methods as an alternative tool to accurately recognize different compositions of similar battery materials regardless of their physical properties or manufacturer. Such applications are of interest for the real-time digitalization of battery components and identification in automated manufacturing and recycling plant designs.
Full article
►▼
Show Figures
Open AccessArticle
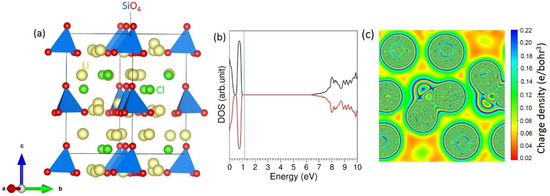
DFT Modelling of Li6SiO4Cl2 Electrolyte Material for Li-Ion Batteries
by
Navaratnarajah Kuganathan
Cited by 2 | Viewed by 2966
Abstract
There is significant interest in finding a promising lithium-containing oxide that can act as a solid electrolyte in a rechargeable lithium-ion battery. Li
6SiO
4Cl
2 is a candidate electrolyte material which was recently characterized using both experimental and computational techniques.
[...] Read more.
There is significant interest in finding a promising lithium-containing oxide that can act as a solid electrolyte in a rechargeable lithium-ion battery. Li
6SiO
4Cl
2 is a candidate electrolyte material which was recently characterized using both experimental and computational techniques. In this study, density functional theory simulation was used to examine the intrinsic defects, solution of promising isovalent and aliovalent dopants, possible reaction routes for the formation of Li
6SiO
4Cl
2, and the feasibility of incorporating additional Li in this material. The results revealed that the O–Cl anti-site cluster was the lowest energy defect in this material. The LiCl Schottky was the second lowest energy defect process, and the Li Frenkel was higher—only by 0.06 eV—than the LiCl Schottky. The candidate dopants on the Li, Si and Cl were Na, Ge and F, respectively. Substituting Al on the Si site was an efficient way of increasing the amount of Li in this material. Incorporation of extra Li (up to three) was considered and this process was endothermic. Different chemical reaction routes were constructed and their reaction energies were calculated to predict the feasibility of the formation of Li
6SiO
4Cl
2. The formation of Li
6SiO
4Cl
2 from constituent elements (Li, Si O
2 and Cl
2) is thermodynamically feasible.
Full article
►▼
Show Figures
Open AccessArticle
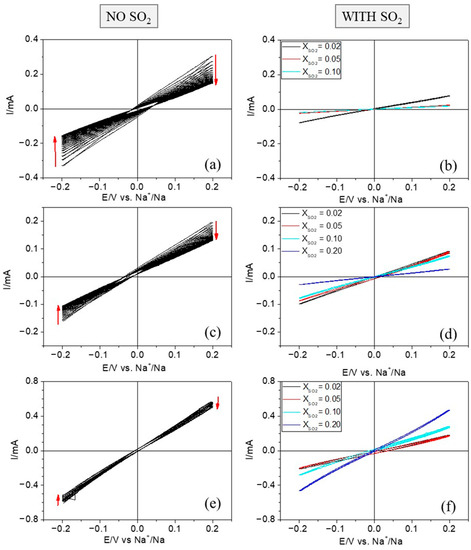
Sulfur Dioxide and Sulfolane as Additives in Organic Electrolytes to Develop Room-Temperature Sodium Batteries
by
Débora Ruiz-Martínez and Roberto Gómez
Cited by 3 | Viewed by 2737
Abstract
Sodium metal anodes have attracted great attention for the development of a next generation of high-energy batteries because of their high theoretical capacity (1166 mAh·g
−1), low redox potential (−2.71 V vs. SHE), and abundance. However, sodium reacts with most of the
[...] Read more.
Sodium metal anodes have attracted great attention for the development of a next generation of high-energy batteries because of their high theoretical capacity (1166 mAh·g
−1), low redox potential (−2.71 V vs. SHE), and abundance. However, sodium reacts with most of the liquid electrolytes described to date and it has the shortcoming of dendrite formation during sodium deposition. Several strategies have been proposed to overcome these issues, including the incorporation of electrolyte additives. This work reports on the use of SO
2 and sulfolane as additives in organic electrolytes to modify the sodium–electrolyte interphase, making the sodium plating/stripping process more robust. Not only is the process more stable in the case of sodium metal anodes, but also the use of copper substrates is enabled. In fact, high-quality sodium films on copper have been attained by adding small mole fractions of the additives, which paves the way for the development of anode-free batteries. In a general vein, this work stresses the importance of researching on compatible and cost-effective additives that can be easily implemented in practice.
Full article
►▼
Show Figures
Open AccessArticle
Design of Perovskite-Type Fluorides Cathodes for Na-ion Batteries: Correlation between Structure and Transport
by
Michele Montalbano, Daniele Callegari, Umberto Anselmi Tamburini and Cristina Tealdi
Cited by 1 | Viewed by 1741
Abstract
Transition metal-based sodium fluoro-perovskite of general formula NaMF
3 (M = Fe, Mn, and Co) were investigated as cathode materials for rechargeable Na-ion batteries. Preliminary results indicated Na-ion reversible intercalation but highlighted the need to find optimization strategies to improve conductivity and to
[...] Read more.
Transition metal-based sodium fluoro-perovskite of general formula NaMF
3 (M = Fe, Mn, and Co) were investigated as cathode materials for rechargeable Na-ion batteries. Preliminary results indicated Na-ion reversible intercalation but highlighted the need to find optimization strategies to improve conductivity and to modulate the operating voltages within experimentally accessible electrolytes’ stability windows, in order to fully exploit their potential as high-voltage cathodes. In this study, we combined experimental and computational techniques to investigate structures, defects, and intercalation properties of the NaFe
1-xMn
xF
3 and NaCo
1-xMn
xF
3 systems. Through the use of a simple solvothermal synthesis, we demonstrated the possibility to modulate the sample’s morphology in order to obtain fine and dispersed powder samples. The structural results indicated the formations of two solid solutions with a perovskite structure over the entire compositional range investigated. Atomistic simulations suggested that Na-ion diffusion in these systems was characterized by relatively high migration barriers and it was likely to follow three-dimensional paths, thus limiting the effect of anti-site defects. The correlation between structural and computational data highlighted the possibility to modulate both ionic and electronic conductivity as a function of the composition.
Full article
►▼
Show Figures
Open AccessArticle
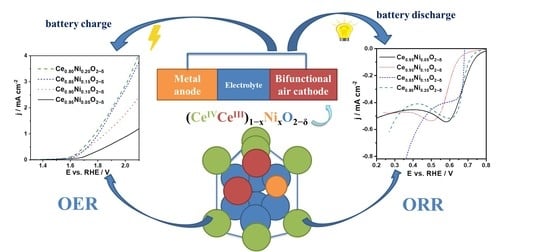
Nickel-Doped Ceria Bifunctional Electrocatalysts for Oxygen Reduction and Evolution in Alkaline Media
by
Jadranka Milikić, Rodolfo O. Fuentes, Julia E. Tasca, Diogo M. F. Santos, Biljana Šljukić and Filipe M. L. Figueiredo
Cited by 7 | Viewed by 1825
Abstract
Nickel-doped ceria (Ce
1−xNi
xO
2−δ) nanopowders (7 to 5 nm in size) synthesized by the cation complexation method with 5, 10, 15, and 20 Ni at.% are studied with respect to their electrochemical activity for the oxygen reduction (ORR)
[...] Read more.
Nickel-doped ceria (Ce
1−xNi
xO
2−δ) nanopowders (7 to 5 nm in size) synthesized by the cation complexation method with 5, 10, 15, and 20 Ni at.% are studied with respect to their electrochemical activity for the oxygen reduction (ORR) and oxygen evolution (OER) reactions in alkaline medium. One finds good bifunctional electrocatalytic activity of the four Ce
1−xNi
xO
2−δ electrocatalysts. The Tafel analysis of the ORR in the 0.57–0.78 V vs. RHE potential window leads to slopes in the 70–108 mV dec
−1 range. The number of electrons exchanged during ORR is between 2 and 2.7. The OER Tafel slopes are determined to be in the range 192 –281 mV dec
−1. OER activation energies are found to range between 28 and 43 kJ mol
−1. The specific capacitance of Ce
1−xNi
xO
2−δ electrocatalysts measured at a scan rate of 100 mV s
−1 varies between 0.7 and 1.4 Fg
−1. The results demonstrate that Ce
1−xNi
xO
2−δ nanopowders can act as bifunctional electrocatalysts for ORR/OER for potential application in the oxygen electrode of devices such as rechargeable metal–air batteries.
Full article
►▼
Show Figures
Open AccessArticle
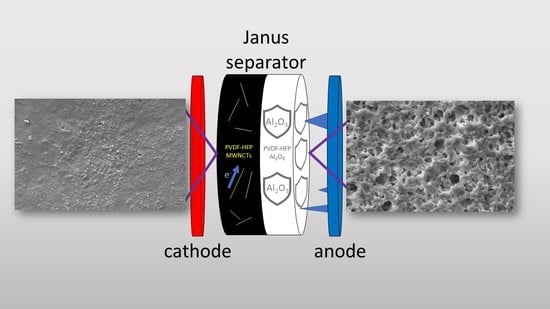
Nanocomposite Janus Gel Polymer Electrolytes for Lithium Metal Batteries
by
Riccardo Morina, Rebecca Baroni, Daniele Callegari, Eliana Quartarone and Piercarlo Mustarelli
Cited by 2 | Viewed by 2084
Abstract
Lithium metal batteries (LMBs) are a key product for sustainable and efficient electric transport. Long-life and safe LMBs require the development of solid or semisolid (e.g., gel polymer) electrolytes capable of blocking lithium dendrites. In this context, Janus double-faced membranes (JMs) offer interesting
[...] Read more.
Lithium metal batteries (LMBs) are a key product for sustainable and efficient electric transport. Long-life and safe LMBs require the development of solid or semisolid (e.g., gel polymer) electrolytes capable of blocking lithium dendrites. In this context, Janus double-faced membranes (JMs) offer interesting perspectives, as they allow for modulating the properties of each side according to specific requests. In this paper, we report on facile fabrication via the solvent casting of JMs based on poly(vinylidene fluoride hexafluoropropylene) (PVDF-HFP). Here, an electronically insulating layer containing Al
2O
3 is in contact with the anode, whereas a mixed ionically–electronically conducting layer containing Al
2O
3, carbon nanotubes, and Super P carbon black is in contact with the cathode. We also investigate the role of the JM thickness and show that a 40 μm membrane allows for ~45% of the specific nominal capacity at 2C with Coulombic efficiency of ~100%. The proposed JMs are very promising for LMBs.
Full article
►▼
Show Figures
Open AccessArticle
A True Non-Newtonian Electrolyte for Rechargeable Hybrid Aqueous Battery
by
Tuan K. A. Hoang, Longyan Li, Jian Zhi, The Nam Long Doan, Wenhan Dong, Xiaoxiao Huang, Junhong Ma, Yahong Xie, Menglei Chang and P. Chen
Viewed by 2090
Abstract
The rechargeable aqueous hybrid battery is a unique system in which the Li-ion mechanism dominates the cathode while the first-order metal reaction of stripping/depositing regulates the anode. This battery inherits the advantages of the low-cost anode while possessing the capability of the Li-ion
[...] Read more.
The rechargeable aqueous hybrid battery is a unique system in which the Li-ion mechanism dominates the cathode while the first-order metal reaction of stripping/depositing regulates the anode. This battery inherits the advantages of the low-cost anode while possessing the capability of the Li-ion cathode. One of the major challenges is to design a proper electrolyte to nourish such strengths and alleviate the downsides, because two different mechanisms are functioning separately at the node–electrolyte and the cathode–electrolyte interfaces. In this work, we design a non-Newtonian electrolyte which offers many advantages for a Zn/LiMn
2O
4 battery. The corrosion is kept low while almost non-dendritic zinc deposition is confirmed by chronoamperometry and ex situ microscopy. The gel strength and gelling duration of such non-Newtonian electrolytes can be controlled. The ionic conductivity of such gels can reach 60 mS⋅cm
−1. The battery exhibits reduced self-discharge, 6–10% higher specific discharge capacity than the aqueous reference battery, high rate capability, nearly 80% capacity retention after 1000 cycles, and about 100 mAh⋅g
−1 of specific discharge capacity at cycle No. 1000th. Negligible amorphization on the cathode surface and no passivation on the anode surface are observed after 1000 cycles, evidenced by X-ray diffraction and scanning electron microscopy on the post-run battery electrodes.
Full article
►▼
Show Figures
Open AccessArticle
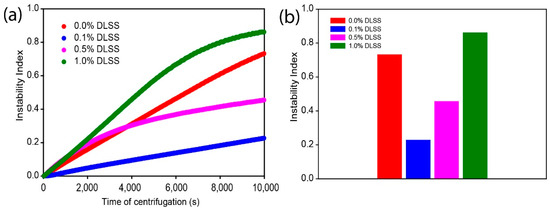
Upgrading the Properties of Ceramic-Coated Separators for Lithium Secondary Batteries by Changing the Mixing Order of the Water-Based Ceramic Slurry Components
by
Ssendagire Kennedy, Jeong-Tae Kim, Yong Min Lee, Isheunesu Phiri and Sun-Yul Ryou
Cited by 6 | Viewed by 3663
Abstract
Developing uniform ceramic-coated separators in high-energy Li secondary batteries has been a challenging task because aqueous ceramic coating slurries have poor dispersion stability and coating quality on the hydrophobic surfaces of polyolefin separators. In this study, we develop a simple but effective strategy
[...] Read more.
Developing uniform ceramic-coated separators in high-energy Li secondary batteries has been a challenging task because aqueous ceramic coating slurries have poor dispersion stability and coating quality on the hydrophobic surfaces of polyolefin separators. In this study, we develop a simple but effective strategy for improving the dispersion stability of aqueous ceramic coating slurries by changing the mixing order of the ceramic slurry components. The aqueous ceramic coating slurry comprises ceramics (Al
2O
3), polymeric binders (sodium carboxymethyl cellulose, CMC), surfactants (disodium laureth sulfosuccinate, DLSS), and water. The interaction between the ceramic slurry components is studied by changing the mixing order of the ceramic slurry components and quantitatively evaluating the dispersion stability of the ceramic coating slurry using a Lumisizer. In the optimized mixing sequence, Al
2O
3 and DLSS premixed in aqueous Al
2O
3-DLSS micelles through strong surface interactions, and they repel each other due to steric repulsion. The addition of CMC in this state does not compromise the dispersion stability of aqueous ceramic coating slurries and enables uniform ceramic coating on polyethylene (PE) separators. The prepared Al
2O
3 ceramic-coated separators (Al
2O
3–CCSs) exhibit improved physical properties, such as high wettability electrolyte uptake and ionic conductivity, compared to the bare PE separators. Furthermore, Al
2O
3–CCSs exhibit improved electrochemical performance, such as rate capability and cycling performance. The half cells (LiMn
2O
4/Li metal) comprising Al
2O
3–CCSs retain 90.4% (88.4 mAh g
−1) of initial discharge capacity after 150 cycles, while 27.6% (26.4 mAh g
−1) for bare PE. Furthermore, the full cells (LiMn
2O
4/graphite) consisting of Al
2O
3–CCSs exhibit 69.8% (72.2 mAh g
−1) of the initial discharge capacity and 24.9% (25.0 mAh g
−1) for bare PE after 1150 cycles.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
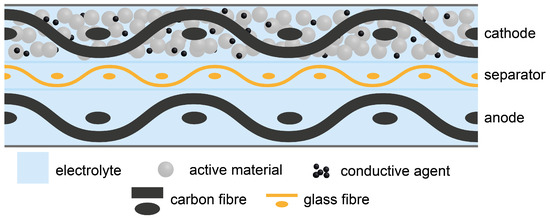
Production and Characterisation of Fibre-Reinforced All-Solid-State Electrodes and Separator for the Application in Structural Batteries
by
Daniel Vogt, Peter Michalowski and Arno Kwade
Cited by 4 | Viewed by 2968
|
Correction
Abstract
The electrification of the air transport sector demands for an energy storage that adds as little volume and weight to the overall system as possible. Regarding this so-called structural battery, composites enable the storage of electrical energy in commonly used load bearing fibre
[...] Read more.
The electrification of the air transport sector demands for an energy storage that adds as little volume and weight to the overall system as possible. Regarding this so-called structural battery, composites enable the storage of electrical energy in commonly used load bearing fibre composite structures. A structural battery composite can store electrical energy while bearing mechanical loads, thus reducing parasitic mass and volume. In this study, structural cathodes were prepared by slurry coating carbon fibres with lithium iron phosphate (LFP), polyethylene oxide (PEO), lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) and carbon black. For the structural anodes, the carbon fibres were utilised as active material and slurry coated with PEO and LiTFSI. These structural electrodes as well as a structural separator were characterised by electrochemical cycling. With 139
, the structural cathodes demonstrated good utilisation of the active material. The carbon fibres used in the anode exhibited capacities of up to 92
. High irreversible lithium losses were observed, which are attributed to the poor electrolyte wetting behaviour of the carbon fibres. A structural battery demonstrator with a lithium metal anode was realised and reached a maximum specific energy of 64
with respect to electrode and separator weight.
Full article
►▼
Show Figures
Planned Papers
The below list represents only planned manuscripts. Some of these
manuscripts have not been received by the Editorial Office yet. Papers
submitted to MDPI journals are subject to peer-review.
Title: Structural and dynamic properties of Li – pyrrolidinium based ionic liquids from a Molecular Dynamics Approach
Authors: Michele A. Salvador; Rita Maji; Alice Ruini; Elena Degoli; Francesco Rossella; Rita Magri
Affiliation: Dipartimento di Scienze Fisiche, Informatiche e Matematiche, Università di Modena e Reggio Emilia via Campi 213/a - 41125 Modena, Italy
Abstract: Pyrrolidinium-based ionic liquids have been proposed as electrolyte components in lithium-ion
batteries (LiB), and their performance is related to the mobility of the Li-ions, thus closely affected by
the Li-ion local environment. In this study we used classical molecular dynamics with a well
established set of forcefield parameters to understand how the structural properties vary with the
cation - anion combinations. We consider ionic liquids obtained by combining pyrrolidinium cations
(Pyr1X: X=1..6) with the anions: chloride (Cl), tetrafluoroborate (BF4), hexafluorophosphate (PF6),
and bis(trifluoromethane)sulfonimide (TFSI), We also analyzed the variations of these properties with
the addition of different fractions of lithium, and investigated the properties related to the Li
conductivity in these electrolyte solutions that would allow them to be applied in LiB. For the neat
ionic liquids (0.0 fraction of Li) the density values vary more significantly with the size of the anion
than with the size of the cation lateral chain, at least for chains up to 6 carbon atoms. This trend is
preserved when we replace a fraction of the pyrrolidinium cations by lithium ions. Furthermore, for
each ionic liquid, the density values increase as we increase the fraction of Li ions in the system.
Analyzing pair correlation functions, we observed that the increase in the amount of lithium
practically does not affect the structure of the cations, while strongly influences the structure of the
anions and also the cation-anion interaction for systems with TFSI. Mapping the Li-anion distances
of different systems, we could understand the formation of clusters, which decreases the mobility of
lithium. This work provided us with a broader understanding of the role of each species in the ionic
liquid - lithium electrolyte solution in determining the structural properties, which eventually will also
affect the dynamic properties.
Title: NMC Cathode Materials: Effect of Processing Parameters on Their Functional Properties
Authors: Nahirniak S. 1,*; Guney E. 2; Saini N. 1; Lontio Fomekong 3; R., Yuca N. 2; Saruhan B. 1
Affiliation: 1. Institute of Materials Research, German Aerospace Center (DLR), Linder Hoehe 51147, Cologne, Germany;
2. ENWAIR Energy Technologies Corporation, Maslak, Istanbul 34469, Turkey;
3. Laboratory of Material Chemistry, Higher Teacher Training College, University of Yaounde I, PO. BOX 47 Yaounde, Cameroon
Abstract: Lithium-Nickel-Manganese-Cobalt-Oxide based cathode materials have proven to yield enhanced specific capacity and thermal stability thus, can outperform lithium iron phosphate cathodes in many areas, particularly in terms of operational voltage in which LFP-based Li-ion batteries can only output voltages below 3.4 V and suffer from high rates of self-discharge. However, due to the issues with their thermal instability at the fully charged state and insufficient cycle life, researchers are forced to explore approaches to improve these for enhancement of the overall performance of cathodes. In this work, Ni-rich NMC cathode particles have been synthesized via a cost efficient and easy process-controlled co-precipitation method using different co-precipitation agents such as oxalic succinic and malonic acids. SEM, EDX and XRD characterizations of the obtained powders have been performed in order to study their morphology and phase composition. Furthermore, the effect of co-precipitation agent on electrochemical performance has been studied by means of CV, CCD and EIS measurements. It was shown that, the differences in the phase characteristics in the NMC powders, produced using various co-precipitation agents, affect primarily the electrochemical performance of the obtained cells. Particle morphology appears to have secondary effect.