Seasonal Influence on Volatile Composition of Psidium friedrichsthalianum Leaves, Sampled in the Brazilian Amazon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Climatic Data
2.2. Essential Oil and Volatile Concentrate Extraction
2.3. Oils and Volatile Concentrate Composition Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Seasonal Effect on Oil Yields
3.2. Seasonal Effect on P. friedrichsthalianum Oil Composition
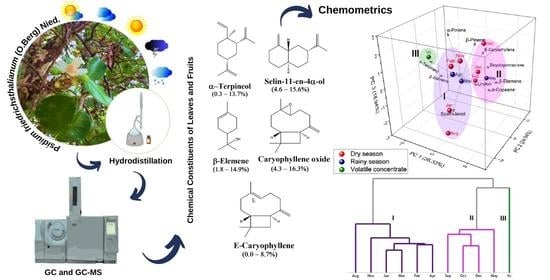
3.3. Multivariate Analysis of P. friedrichsthalianum
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, R.C.E.; Costa, J.S.D.; Figueiredo, R.O.D.; Setzer, W.N.; Silva, J.K.R.D.; Maia, J.G.S.; Figueiredo, P.L.B. Monoterpenes and Sesquiterpenes of Essential Oils from Psidium Species and Their Biological Properties. Molecules 2021, 26, 965. [Google Scholar] [CrossRef]
- Psidium, L. GBIF Backbone Taxonomia. In Conjunto de Dados Da Lista de Verificação; NoSecretariado Do GBIF: Palikir, Micronesia, 2022. [Google Scholar]
- Da Costa, J.S.; Andrade, W.M.S.; de Figueiredo, R.O.; Santos, P.V.L.; Freitas, J.J.d.S.; Setzer, W.N.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Chemical Composition and Variability of the Volatile Components of Myrciaria Species Growing in the Amazon Region. Molecules 2022, 27, 2234. [Google Scholar] [CrossRef]
- Juárez-Vázquez, M.D.C.; Carranza-Álvarez, C.; Alonso-Castro, A.J.; González-Alcaraz, V.F.; Bravo-Acevedo, E.; Chamarro-Tinajero, F.J.; Solano, E. Ethnobotany of Medicinal Plants Used in Xalpatlahuac, Guerrero, México. J. Ethnopharmacol. 2013, 148, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, K.F.; Moreira, F.M.F.; Alencar Santos, J.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Vieira, M.d.C.; Góis Ruiz, A.L.T.; Ann Foglio, M.; de Carvalho, J.E.; et al. Antioxidant, Anti-Inflammatory, Antiproliferative and Antimycobacterial Activities of the Essential Oil of Psidium guineense Sw. and Spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef]
- Santos, P.V.L.; Ellen de Nazaré, S.; de Sousa Barroso, A.; Mourão, R.H.V.; Setzer, W.N.; da Silva, J.K.; do Nascimento, W.M.O.; da Costa, J.S.; Figueiredo, P.L.B. Chemometric Analysis of the Seasonal Variation in the Essential Oil Composition of Psidium acutangulum Growing in the Brazilian Amazon. Biochem. Syst. Ecol. 2022, 105, 104528. [Google Scholar] [CrossRef]
- Jerônimo, L.B.; da Costa, J.S.; Pinto, L.C.; Montenegro, R.C.; Setzer, W.N.; Mourão, R.H.V.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Antioxidant and Cytotoxic Activities of Myrtaceae Essential Oils Rich in Terpenoids from Brazil. Nat. Prod. Commun. 2021, 16, 1934578X2199615. [Google Scholar] [CrossRef]
- Gentil, D.F.d.O.; Ferreira, S.A.d.N.; Rebouças, E.R. Germination of Psidium friedrichsthalianum (O. Berg) Nied. Seeds under Different Temperature and Storage Conditions. J. Seed Sci. 2018, 40, 246–252. [Google Scholar] [CrossRef]
- Montoya-Arroyo, A.; Díaz, C.; Vaillant, F.; Tamayo-Castillo, G. Oral Administration of Costa Rican Guava (Psidium friedrichsthalianum) Juice Induces Changes in Urinary Excretion of Energy-Related Compounds in Wistar Rats Determined by 1H NMR. NFS J. 2020, 20, 48–57. [Google Scholar] [CrossRef]
- Cuadrado-Silva, C.T.; Pozo-Bayón, M.Á.; Osorio, C. Targeted Metabolomic Analysis of Polyphenols with Antioxidant Activity in Sour Guava (Psidium friedrichsthalianum Nied.) Fruit. Molecules 2017, 22, 11. [Google Scholar] [CrossRef] [Green Version]
- Miranda-Cruz, E.; Espinosa-Moreno, J.; Centurión-Hidalgo, D.; Velázquez-Martínez, J.R.; Alor-Chávez, M.d.J. Antimicrobial Activity of Psidium friedrichsthalianum L., Pterocarpus hayesii L., Tynanthus guatemalensis L. and Spondias purpurea L. Extracts. Bol. Latinoam. Caribe Plantas Med. Aromat. 2012, 11, 354–361. [Google Scholar]
- Van Den Dool, H.; Kratz, P. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Mondello, L. FFNSC 2: Flavors and Fragrances of Natural and Synthetic Compounds, Mass Spectral Database; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011; ISBN 1118145836. [Google Scholar]
- Da Cruz, E.d.N.S.; Peixoto, L.d.S.; da Costa, J.S.; Mourão, R.H.V.; do Nascimento, W.M.O.; Maia, J.G.S.; Setzer, W.N.; da Silva, J.K.; Figueiredo, P.L.B. Seasonal Variability of a Caryophyllane Chemotype Essential Oil of Eugenia patrisii Vahl Occurring in the Brazilian Amazon. Molecules 2022, 27, 2417. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.d.S.P.; Santos da Cruz, E.d.N.; de Araújo Guimarães, B.; Setzer, W.N.; Veras Mourão, R.H.; do Rosário da Silva, J.K.; Silva da Costa, J.; Baia Figueiredo, P.L. Chemometric Analysis of the Seasonal Variation in the Essential Oil Composition and Antioxidant Activity of a New Geraniol Chemotype of Lippia alba (Mill.) N.E.Br. Ex Britton & P. Wilson from the Brazilian Amazon. Biochem. Syst. Ecol. 2022, 105, 104503. [Google Scholar] [CrossRef]
- De Loureiro, R.S.; Saraiva, J.M.; Saraiva, I.; Senna, R.C.; Fredó, A.S. Estudo Dos Eventos Extremos de Precipitação Ocorridos Em 2009 No Estado Do Pará. Rev. Bras. Meteorol. 2014, 29, 83–94. [Google Scholar] [CrossRef]
- Da Costa, J.S.; da Cruz, E.d.N.; Setzer, W.N.; da Silva, J.K.d.R.; Maia, J.G.S.; Figueiredo, P.L.B. Essentials Oils from Brazilian Eugenia and Syzygium Species and Their Biological Activities. Biomolecules 2020, 10, 1155. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, J.S.; Barroso, A.S.; Mourão, R.H.V.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Seasonal and Antioxidant Evaluation of Essential Oil from Eugenia uniflora L., Curzerene-Rich, Thermally Produced In Situ. Biomolecules 2020, 10, 328. [Google Scholar] [CrossRef] [Green Version]
- De Macêdo, D.G.; Souza, M.M.A.; Morais-Braga, M.F.B.; Coutinho, H.D.M.; dos Santos, A.T.L.; da Cruz, R.P.; da Costa, J.G.M.; Rodrigues, F.F.G.; Quintans-junior, L.J.; da Silva Almeida, J.R.G.; et al. Effect of Seasonality on Chemical Profile and Antifungal Activity of Essential Oil Isolated from Leaves Psidium salutare (Kunth) O. Berg. PeerJ 2018, 6, e5476. [Google Scholar] [CrossRef] [Green Version]
- Tucker, A.O.; Maciarello, M.J.; Landrum, L.R. Volatile Leaf Oils of American Myrtaceae. III. Psidium cattleianum Sabine, P. friedrichsthalianum (Berg) Niedenzu, P. guajava L., P. guineense Sw., and P. sartorianum (Berg) Niedenzu. J. Essent. Oil Res. 1995, 7, 187–190. [Google Scholar] [CrossRef]
- Pino, J.A.; Marbot, R.; Vázquez, C. Characterization of Volatiles in Costa Rican Guava [Psidium friedrichsthalianum (Berg) Niedenzu] Fruit. J. Agric. Food Chem. 2002, 50, 6023–6026. [Google Scholar] [CrossRef]
- Vasconcelos, L.C.; de Souza Santos, E.; de Oliveira Bernardes, C.; da Silva Ferreira, M.F.; Ferreira, A.; Tuler, A.C.; Carvalho, J.A.M.; Pinheiro, P.F.; Praça-Fontes, M.M. Phytochemical Analysis and Effect of the Essential Oil of Psidium L. Species on the Initial Development and Mitotic Activity of Plants. Environ. Sci. Pollut. Res. 2019, 26, 26216–26228. [Google Scholar] [CrossRef]
- Flores, G.; Dastmalchi, K.; Wu, S.B.; Whalen, K.; Dabo, A.J.; Reynertson, K.A.; Foronjy, R.F.; D’Armiento, J.M.; Kennelly, E.J. Phenolic-Rich Extract from the Costa Rican Guava (Psidium friedrichsthalianum) Pulp with Antioxidant and Anti-Inflammatory Activity. Potential for COPD Therapy. Food Chem. 2013, 141, 889–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas-Garbanzo, C.; Rodríguez, L.; Pérez, A.M.; Mayorga-Gross, A.L.; Vásquez-Chaves, V.; Fuentes, E.; Palomo, I. Anti-Platelet Activity and Chemical Characterization by UPLC-DAD-ESI-QTOF-MS of the Main Polyphenols in Extracts from Psidium Leaves and Fruits. Food Res. Int. 2021, 141, 110070. [Google Scholar] [CrossRef] [PubMed]




| Oil Yield/Components | Temperature | Humidity | Insolation | Precipitation |
|---|---|---|---|---|
| Oil yield | −0.32 | 0.63 * | −0.70 * | 0.57 |
| Caryophyllene oxide | 0.51 | 0.57 | 0.16 | 0.63 * |
| β-Pinene | 0.26 | 0.36 | −0.24 | −0.43 |
| α-Pinene | −0.44 | 0.33 | −0.70 * | 0.01 |
| β-Elemene | 0.86 * | 0.88 * | 0.44 | 0.60 * |
| α-Terpineol | −0.99 * | −0.99 * | −0.66 * | −0.65 * |
| β-Selinene | −0.75 * | −0.72 * | −0.59 * | −0.41 |
| Selin-11-en-4-α-ol | −0.22 | −0.27 | 0.16 | −0.05 |
| Monoterpene hydrocarbons | −0.29 | −0.26 | −0.49 | −0.32 |
| Oxygenated monoterpenes | −0.79 * | −0.78 * | −0.47 | −0.38 |
| Sesquiterpene hydrocarbons | 0.41 | 0.36 | 0.36 | 0.10 |
| Oxygenated sesquiterpenes | 0.28 | 0.35 | 0.13 | 0.42 |
| RIC | RIL | Oil Yield (%) | 0.5 | 0.5 | 0.4 | 0.6 | 0.6 | 0.5 | 0.7 | 0.8 | 0.8 | 0.8 | Vc (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Month/Constituents (%) | Aug | Sep | Oct | Nov | Dec | Jan | Feb | Mar | Apr | May | |||
| 926 | 924 a | α-Thujene | n.d. | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | n.d. |
| 934 | 932 a | α-Pinene | 6.3 | 9.1 | 14.0 | 17.2 | 11.5 | 10.8 | 18.0 | 12.9 | 14.9 | 10.8 | 17.6 |
| 947 | 945 a | α-Fenchene | n.d. | 0.1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.1 |
| 949 | 946 a | Camphene | n.d. | 0.1 | n.d. | n.d. | n.d. | 0.1 | n.d. | n.d. | 0.1 | n.d. | 0.2 |
| 974 | 969 a | Sabinene | 0.9 | 0.2 | 0.5 | 0.6 | 0.5 | 0.6 | 0.8 | 0.5 | 0.5 | 0.4 | n.d. |
| 978 | 974 a | β-Pinene | 4.8 | 6.9 | 11.0 | 11.9 | 9.5 | 8.9 | 13.4 | 9.8 | 10.5 | 8.4 | 7.1 |
| 991 | 988 a | Myrcene | n.d. | 0.5 | 1.1 | 0.5 | 1.0 | 0.5 | 0.6 | 0.7 | 0.6 | 0.8 | 1.1 |
| 1017 | 1014 a | α-Terpinene | n.d. | 0.1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.2 |
| 1024 | 1020 a | p-Cymene | 0.4 | 0.4 | 0.1 | 0.2 | n.d. | 0.3 | 0.2 | 0.2 | 0.2 | 0.1 | n.d. |
| 1028 | 1024 a | Limonene | 0.7 | 1.4 | 1.5 | 1.4 | 1.4 | 1.3 | 1.5 | 1.3 | 1.2 | 1.2 | 2.2 |
| 1031 | 1026 a | 1,8-Cineole | n.d. | n.d. | n.d. | n.d. | 0.1 | n.d. | n.d. | 0.2 | n.d. | n.d. | n.d. |
| 1046 | 1044 a | E-β-Ocimene | n.d. | n.d. | 0.1 | n.d. | 0.1 | n.d. | n.d. | n.d. | n.d. | 0.1 | 0.2 |
| 1058 | 1054 | γ-Terpinene | 0.1 | 0.1 | n.d. | n.d. | 0.1 | n.d. | n.d. | n.d. | n.d. | 0.1 | 0.3 |
| 1072 | 1067 a | cis-Linalool oxide (furanoid) | 0.1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.2 | n.d. |
| 1088 | 1084 a | trans-Linalool oxide (furanoid) | 0.2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.1 | n.d. |
| 1089 | 1086 a | Terpinolene | n.d. | 0.2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 2.2 |
| 1100 | 1095 a | Linalool | 0.5 | 0.1 | 0.4 | 0.3 | 0.5 | 1.7 | 0.9 | 0.7 | 0.6 | 7.1 | 0.2 |
| 1113 | 1114 a | endo-Fenchol | 0.1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.5 |
| 1126 | 1122 a | α-Campholenal | 0.3 | n.d. | n.d. | 0.1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1137 | 1135 a | Nopilone | 0.2 | n.d. | n.d. | 0.1 | n.d. | 0.1 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1139 | 1135 a | trans-Pinocarveol | 1.3 | n.d. | 0.1 | 0.7 | 0.1 | 0.6 | 0.3 | 0.3 | 0.6 | n.d. | n.d. |
| 1145 | 1140 a | trans-Verbenol | 1.5 | n.d. | n.d. | 0.4 | n.d. | 0.3 | n.d. | 0.1 | 0.3 | n.d. | n.d. |
| 1162 | 1160 a | Pinocarvone | 0.7 | n.d. | n.d. | 0.2 | n.d. | 0.4 | 0.2 | 0.2 | 0.4 | n.d. | n.d. |
| 1166 | 1165 a | Borneol | 0.1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.3 |
| 1177 | 1174 a | Terpinen-4-ol | 0.1 | 0.3 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | n.d. | 0.7 |
| 1191 | 1186 a | α-Terpineol | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.4 | 13.7 |
| 1196 | 1195 a | Myrtenal | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.3 | 0.3 | 0.5 | n.d. | n.d. |
| 1197 | 1197 b | Myrtenol | 1.4 | n.d. | n.d. | 0.7 | n.d. | 0.7 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1209 | 1204 a | Verbenone | 0.9 | n.d. | n.d. | 0.1 | n.d. | 0.2 | n.d. | n.d. | 0.1 | n.d. | n.d. |
| 1219 | 1215 a | trans-Carveol | 0.2 | n.d. | n.d. | 0.1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1221 | 1218 a | endo-Fenchyl acetate | 0.1 | 0.1 | n.d. | n.d. | 0.1 | 0.1 | n.d. | 0.1 | 0.1 | n.d. | n.d. |
| 1243 | 1241 b | Methyl phenethyl ketone | n.d. | 0.1 | 0.2 | 0.3 | 0.1 | 0.1 | n.d. | 0.2 | 0.3 | n.d. | n.d. |
| 1286 | 1287 a | Bornyl acetate | 0.4 | 0.2 | 0.2 | 0.2 | 0.2 | 0.4 | 0.1 | 0.3 | 0.3 | 0.1 | n.d. |
| 1300 | 1298 a | trans-Pinocarvyl acetate | n.d. | 0.1 | 0.1 | 0.1 | 0.1 | n.d. | 0.1 | 0.2 | 0.2 | 0.1 | n.d. |
| 1323 | 1325 a | p-Mentha-1,4-dien-7-ol | 1.0 | n.d. | n.d. | 0.2 | n.d. | n.d. | n.d. | 0.1 | n.d. | n.d. | n.d. |
| 1326 | 1326 b | Myrtenyl acetate | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.2 | 0.2 | 0.1 | n.d. |
| 1338 | 1335 a | δ-Elemene | n.d. | 0.3 | 0.3 | 0.1 | 0.4 | n.d. | n.d. | 0.1 | n.d. | 0.3 | n.d. |
| 1351 | 1345 a | α-Cubebene | n.d. | 0.1 | n.d. | 0.1 | n.d. | n.d. | n.d. | 0.1 | n.d. | 0.1 | n.d. |
| 1377 | 1374 a | α-Copaene | 2.2 | 2.6 | 2.2 | 3.1 | 2.5 | 2.8 | 2.6 | 2.7 | 2.1 | 2.3 | 0.9 |
| 1385 | 1387 a | β-Bourbonene | 0.4 | n.d. | n.d. | n.d. | n.d. | 0.5 | 0.3 | n.d. | 0.4 | n.d. | n.d. |
| 1394 | 1389 a | β-Elemene | 11.4 | 9.9 | 13.0 | 14.9 | 14.8 | 11.0 | 14.7 | 14.5 | 10.7 | 14.8 | 1.8 |
| 1422 | 1417 a | E-Caryophyllene | n.d. | 8.5 | 8.3 | 3.6 | 8.7 | 1.9 | 4.4 | 6.4 | 3.1 | 8.3 | 5.0 |
| 1430 | 1430 a | β-Copaene | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | n.d. | 0.1 | 0.1 | 0.1 | n.d. |
| 1436 | 1432 a | trans-α-Bergamotene | n.d. | 0.1 | n.d. | 0.2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1440 | 1439 a | Aromadendrene | 0.4 | 0.2 | 0.2 | 0.4 | 0.3 | 0.4 | 0.1 | 0.3 | 0.3 | 0.2 | n.d. |
| 1455 | 1452 a | α-Humulene | n.d. | 1.3 | 1.2 | 0.7 | 1.3 | 0.4 | 0.5 | 1.0 | 0.6 | 1.1 | 0.7 |
| 1458 | 1454 a | E-β-Farnesene | n.d. | 0.1 | n.d. | n.d. | 0.1 | n.d. | n.d. | 0.1 | n.d. | n.d. | n.d. |
| 1462 | 1464 a | 9-epi-E-Caryophyllene | 0.1 | 0.4 | 0.3 | 0.3 | 0.4 | 0.3 | n.d. | 0.3 | 0.2 | 0.3 | n.d. |
| 1477 | 1476 a | Selina-4,11-diene | n.d. | n.d. | n.d. | n.d. | 0.3 | n.d. | n.d. | n.d. | n.d. | n.d. | 2.0 |
| 1478 | 1478 a | γ-Muurolene | 0.6 | 0.7 | 0.6 | 0.8 | 0.5 | 0.8 | 0.5 | 0.8 | 0.7 | 0.6 | n.d. |
| 1482 | 1480 a | Germacrene D | n.d. | 2.6 | 2.4 | n.d. | 2.9 | n.d. | n.d. | 0.4 | n.d. | 2.6 | n.d. |
| 1488 | 1489 a | β-Selinene | 2.2 | 3.7 | 1.3 | 1.7 | 3.0 | 3.4 | 3.0 | 3.3 | 1.3 | 3.4 | 6.0 |
| 1493 | 1493 a | trans-Muurola-4(14),5-diene | n.d. | 0.2 | 0.2 | n.d. | 0.2 | n.d. | n.d. | n.d. | n.d. | 0.1 | n.d. |
| 1496 | 1498 b | epi-Cubebol | 0.6 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 1.0 | n.d. | n.d. |
| 1498 | 1500 a | Bicyclogermacrene | n.d. | 6.1 | 7.3 | n.d. | 6.4 | n.d. | n.d. | n.d. | n.d. | 6.5 | n.d. |
| 1501 | 1500 a | α-Muurolene | 0.2 | 0.5 | 0.5 | 0.3 | 0.4 | 0.4 | 0.1 | 0.3 | 0.3 | 0.3 | n.d. |
| 1506 | 1509 a | α-Bulnesene | n.d. | 0.6 | n.d. | 0.1 | 1.1 | n.d. | n.d. | 0.5 | n.d. | n.d. | n.d. |
| 1516 | 1514 a | Cubebol | n.d. | 0.8 | n.d. | 1.1 | 1.1 | n.d. | n.d. | 1.1 | 1.1 | 0.7 | n.d. |
| 1525 | 1522 a | δ-Cadinene | n.d. | 2.5 | 1.9 | n.d. | 1.9 | n.d. | n.d. | n.d. | n.d. | 1.9 | 1.1 |
| 1534 | 1533 a | trans-Cadina-1,4-diene | n.d. | 0.1 | 0.1 | n.d. | 0.1 | n.d. | n.d. | n.d. | n.d. | 0.1 | n.d. |
| 1539 | 1537 a | α-Cadinene | 0.9 | 0.1 | n.d. | 0.1 | 0.1 | n.d. | n.d. | 0.1 | n.d. | n.d. | n.d. |
| 1542 | 1539 a | α-Copaen-11-ol | n.d. | 0.1 | n.d. | 0.1 | 0.1 | 0.2 | n.d. | 0.1 | 0.1 | n.d. | n.d. |
| 1550 | 1548 a | Elemol | 0.2 | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 | n.d. | 0.2 | 0.2 | 0.1 | n.d. |
| 1558 | 1559 a | Germacrene B | 0.2 | 0.1 | 0.1 | n.d. | 0.2 | 0.2 | n.d. | n.d. | 0.1 | 0.1 | n.d. |
| 1565 | 1561 a | E-Nerolidol | n.d. | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | n.d. | 0.1 | 0.1 | 0.1 | n.d. |
| 1568 | 1566 a | Maaliol | 0.5 | 0.2 | 0.1 | 0.2 | 0.2 | 0.3 | n.d. | 0.2 | 0.2 | 0.2 | n.d. |
| 1571 | 1570 a | Caryophyllenyl alcohol | 0.4 | 0.4 | 0.2 | 0.1 | 0.3 | 0.2 | n.d. | 0.3 | 0.3 | 0.2 | n.d. |
| 1579 | 1577 a | Spathulenol | 5.0 | 2.0 | n.d. | 2.4 | 3.7 | 5.7 | 5.9 | 4.0 | 3.1 | 2.6 | 1.9 |
| 1585 | 1582 a | Caryophyllene oxide | 16.3 | 4.7 | 4.3 | 11.2 | 4.3 | 13.6 | 13.6 | 9.5 | 12.4 | 4.4 | n.d. |
| 1588 | 1590 a | β-Copaen-4α-ol | n.d. | 0.3 | 0.3 | 0.1 | 0.3 | 0.3 | n.d. | 0.3 | 0.3 | 0.2 | n.d. |
| 1593 | 1592 a | Viridiflorol | 1.0 | 0.5 | 0.4 | 0.5 | 0.4 | 0.8 | 0.4 | 0.5 | 0.5 | 0.5 | 2.8 |
| 1595 | 1595 a | Cubeban-11-ol | 0.3 | 0.3 | 0.3 | 0.2 | 0.3 | 0.3 | 0.1 | 0.3 | 0.3 | 0.2 | 0.4 |
| 1599 | 1600 a | Guaiol | n.d. | 0.2 | 0.2 | n.d. | 0.2 | n.d. | n.d. | 0.1 | n.d. | 0.1 | n.d. |
| 1604 | 1602 a | Ledol | n.d. | 0.8 | n.d. | 0.6 | n.d. | 0.6 | 0.3 | 0.8 | 0.7 | 0.6 | n.d. |
| 1610 | 1608 a | Humulene epoxide II | 1.7 | 0.3 | 0.3 | 1.1 | n.d. | 1.2 | 0.7 | 0.9 | 1.0 | 0.2 | n.d. |
| 1619 | 1618 a | Junenol | n.d. | 0.5 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1630 | 1630 a | Muurola-4,10(14)-dien-1β-ol | 0.8 | 1.6 | 1.2 | 0.8 | 1.3 | 1.4 | 1.1 | 1.5 | 1.4 | 1.0 | n.d. |
| 1632 | 1630 a | γ-Eudesmol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 1.7 |
| 1638 | 1636 b | Caryophylla-4(12),8(13)-dien-5β-ol | 0.3 | 0.5 | 0.5 | 0.2 | 0.6 | 0.5 | 0.2 | 0.6 | 0.4 | 0.5 | 0.8 |
| 1644 | 1640 a | epi-α-Muurolol (= τ-Muurolol) | 1.5 | 1.9 | 1.8 | 1.6 | 1.7 | 1.8 | 1.7 | 1.9 | 2.0 | 1.7 | 2.0 |
| 1649 | 1644 b | -Muurolol (= Torreyol) | 1.5 | 1.7 | 1.5 | 1.3 | 1.3 | 1.4 | 1.4 | 1.5 | 1.7 | 1.3 | 1.3 |
| 1658 | 1651 | Selin-11-en-4α-ol | 8.0 | 15.6 | 7.5 | n.d. | 4.6 | 6.8 | 7.6 | 5.3 | 9.1 | 5.5 | 10.0 |
| 1669 | 1668 a | trans-Calamenen-10-ol | 0.1 | n.d. | n.d. | 0.2 | n.d. | 0.2 | n.d. | n.d. | 0.1 | n.d. | n.d. |
| 1676 | 1675 a | Cadalene | 0.4 | 0.1 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.1 | 0.2 | n.d. | n.d. |
| 1677 | 1676 a | Muskatone | 0.6 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1687 | 1685 a | α-Bisabolol | n.d. | 0.3 | n.d. | 0.3 | 0.4 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1691 | 1688 a | Shyobunol | n.d. | n.d. | n.d. | n.d. | 0.3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1691 | 1692 a | Acorenone | 0.6 | n.d. | n.d. | n.d. | n.d. | 0.4 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1738 | 1739 a | Oplopanone | n.d. | n.d. | n.d. | 0.2 | n.d. | 0.2 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Monoterpene hydrocarbons | 13.2 | 19.2 | 28.4 | 31.9 | 24.2 | 22.6 | 34.6 | 25.5 | 28.1 | 22.0 | 31.2 | ||
| Oxygenated monoterpenes | 9.6 | 1.3 | 1.5 | 4.0 | 1.7 | 5.3 | 2.4 | 3.3 | 4.0 | 8.1 | 15.4 | ||
| Sesquiterpene hydrocarbons | 19.1 | 40.9 | 40.0 | 26.5 | 45.7 | 22.2 | 26.2 | 31.1 | 20.1 | 43.1 | 17.5 | ||
| Oxygenated sesquiterpenes | 39.4 | 32.9 | 18.8 | 22.5 | 21.4 | 36.2 | 33.0 | 29.2 | 36.0 | 20.1 | 20.9 | ||
| Total (%) | 81.3 | 94.3 | 88.7 | 84.9 | 93.0 | 86.3 | 96.2 | 89.1 | 88.2 | 93.3 | 85.0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, P.V.L.; da Cruz, E.d.N.S.; Nunes, J.d.A.; Mourão, R.H.V.; do Nascimento, W.M.O.; Maia, J.G.S.; Figueiredo, P.L.B. Seasonal Influence on Volatile Composition of Psidium friedrichsthalianum Leaves, Sampled in the Brazilian Amazon. Horticulturae 2023, 9, 768. https://doi.org/10.3390/horticulturae9070768
Santos PVL, da Cruz EdNS, Nunes JdA, Mourão RHV, do Nascimento WMO, Maia JGS, Figueiredo PLB. Seasonal Influence on Volatile Composition of Psidium friedrichsthalianum Leaves, Sampled in the Brazilian Amazon. Horticulturae. 2023; 9(7):768. https://doi.org/10.3390/horticulturae9070768
Chicago/Turabian StyleSantos, Paulo Vinicius L., Ellen de Nazaré Santos da Cruz, Jennifer de Andrade Nunes, Rosa Helena V. Mourão, Walnice Maria O. do Nascimento, José Guilherme S. Maia, and Pablo Luis B. Figueiredo. 2023. "Seasonal Influence on Volatile Composition of Psidium friedrichsthalianum Leaves, Sampled in the Brazilian Amazon" Horticulturae 9, no. 7: 768. https://doi.org/10.3390/horticulturae9070768