Distinct Impacts of UV-A Light Wavelengths on Nutraceutical and Mineral Contents in Green and Purple Basil Cultivated in a Controlled Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Cultivation
2.2. Lighting Experiments
2.3. Biometric Measurements
2.4. Antioxidant Properties and Phenolic Compounds
2.5. Determination of Carotenoids by HPLC
2.6. Quantification of Ascorbic Acid by HPLC
2.7. Determination of Mineral Elements
2.8. Statistical Analysis
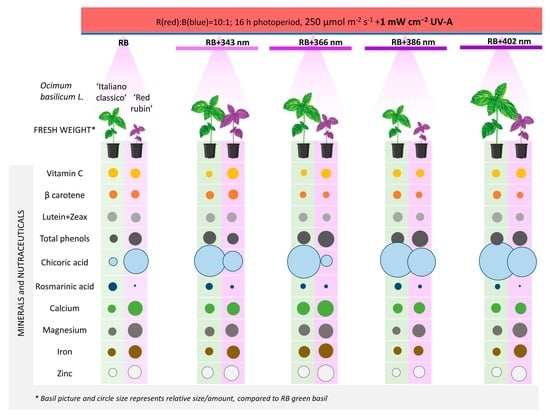
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Variables | Height | Leaf Area | Fresh Weight | DM% | β Carotene | Lutein+ Zea-Xanthin | Ascorbic Acid | Chicoric Acid | Rosmarinic Acid | DPPH | ABTS | Phenols | FRAP | P | Ca | Fe | K | Mg | Mn | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Height | 1 | 0.346 | 0.308 | 0.002 | −0.306 | −0.414 | −0.602 | 0.475 | 0.149 | 0.159 | 0.615 | 0.617 | 0.049 | −0.749 | −0.764 | −0.546 | −0.666 | −0.681 | −0.586 | −0.561 |
| Leaf area | 0.346 | 1 | 0.891 | 0.114 | −0.045 | 0.220 | −0.192 | 0.491 | −0.077 | 0.582 | 0.303 | 0.268 | 0.557 | −0.450 | −0.628 | −0.759 | −0.729 | −0.674 | −0.222 | −0.409 |
| Fresh weight | 0.308 | 0.891 | 1 | 0.060 | −0.052 | 0.203 | −0.206 | 0.551 | 0.016 | 0.471 | 0.342 | 0.232 | 0.607 | −0.478 | −0.625 | −0.686 | −0.692 | −0.653 | −0.245 | −0.447 |
| DM% | 0.002 | 0.114 | 0.060 | 1 | −0.243 | −0.314 | −0.281 | 0.049 | −0.233 | 0.307 | 0.358 | −0.052 | 0.072 | −0.289 | −0.243 | −0.448 | −0.334 | −0.342 | −0.400 | −0.279 |
| β carotene | −0.306 | −0.045 | −0.052 | −0.243 | 1 | 0.734 | 0.684 | −0.277 | 0.599 | 0.255 | −0.379 | −0.424 | 0.116 | 0.405 | 0.270 | 0.206 | 0.138 | 0.139 | 0.264 | −0.068 |
| Lutein + Zeaxanthin | −0.414 | 0.220 | 0.203 | −0.314 | 0.734 | 1 | 0.834 | 0.008 | 0.369 | 0.340 | −0.693 | −0.302 | 0.192 | 0.496 | 0.348 | 0.231 | 0.191 | 0.211 | 0.555 | 0.169 |
| Ascorbic acid | −0.602 | −0.192 | −0.206 | −0.281 | 0.684 | 0.834 | 1 | −0.122 | 0.450 | 0.115 | −0.799 | −0.347 | −0.156 | 0.631 | 0.607 | 0.532 | 0.497 | 0.481 | 0.543 | 0.262 |
| Chicoric acid | 0.475 | 0.491 | 0.551 | 0.049 | −0.277 | 0.008 | −0.122 | 1 | 0.422 | 0.460 | 0.232 | 0.853 | 0.068 | −0.747 | −0.714 | −0.509 | −0.673 | −0.705 | −0.538 | −0.676 |
| Rosmarinic acid | 0.149 | −0.077 | 0.016 | −0.233 | 0.599 | 0.369 | 0.450 | 0.422 | 1 | 0.319 | −0.095 | 0.343 | −0.168 | −0.245 | −0.228 | 0.029 | −0.223 | −0.287 | −0.323 | −0.580 |
| DPPH | 0.159 | 0.582 | 0.471 | 0.307 | 0.255 | 0.340 | 0.115 | 0.460 | 0.319 | 1 | 0.206 | 0.300 | 0.325 | −0.298 | −0.427 | −0.483 | −0.540 | −0.517 | −0.323 | −0.458 |
| ABTS | 0.615 | 0.303 | 0.342 | 0.358 | −0.379 | −0.693 | −0.799 | 0.232 | −0.095 | 0.206 | 1 | 0.372 | 0.371 | −0.747 | −0.721 | −0.670 | −0.667 | −0.674 | −0.790 | −0.587 |
| Phenols | 0.617 | 0.268 | 0.232 | −0.052 | −0.424 | −0.302 | −0.347 | 0.853 | 0.343 | 0.300 | 0.372 | 1 | −0.029 | −0.779 | −0.711 | −0.370 | −0.583 | −0.621 | −0.593 | −0.573 |
| FRAP | 0.049 | 0.557 | 0.607 | 0.072 | 0.116 | 0.192 | −0.156 | 0.068 | −0.168 | 0.325 | 0.371 | −0.029 | 1 | −0.132 | −0.277 | −0.366 | −0.364 | −0.323 | 0.004 | −0.099 |
| P | −0.749 | −0.450 | −0.478 | −0.289 | 0.405 | 0.496 | 0.631 | −0.747 | −0.245 | −0.298 | −0.747 | −0.779 | −0.132 | 1 | 0.952 | 0.770 | 0.887 | 0.918 | 0.893 | 0.850 |
| Ca | −0.764 | −0.628 | −0.625 | −0.243 | 0.270 | 0.348 | 0.607 | −0.714 | −0.228 | −0.427 | −0.721 | −0.711 | −0.277 | 0.952 | 1 | 0.856 | 0.965 | 0.967 | 0.797 | 0.842 |
| Fe | −0.546 | −0.759 | −0.686 | −0.448 | 0.206 | 0.231 | 0.532 | −0.509 | 0.029 | −0.483 | −0.670 | −0.370 | −0.366 | 0.770 | 0.856 | 1 | 0.935 | 0.908 | 0.689 | 0.752 |
| K | −0.666 | −0.729 | −0.692 | −0.334 | 0.138 | 0.191 | 0.497 | −0.673 | −0.223 | −0.540 | −0.667 | −0.583 | −0.364 | 0.887 | 0.965 | 0.935 | 1 | 0.993 | 0.763 | 0.859 |
| Mg | −0.681 | −0.674 | −0.653 | −0.342 | 0.139 | 0.211 | 0.481 | −0.705 | −0.287 | −0.517 | −0.674 | −0.621 | −0.323 | 0.918 | 0.967 | 0.908 | 0.993 | 1 | 0.808 | 0.896 |
| Mn | −0.586 | −0.222 | −0.245 | −0.400 | 0.264 | 0.555 | 0.543 | −0.538 | −0.323 | −0.323 | −0.790 | −0.593 | 0.004 | 0.893 | 0.797 | 0.689 | 0.763 | 0.808 | 1 | 0.864 |
| Zn | −0.561 | −0.409 | −0.447 | −0.279 | −0.068 | 0.169 | 0.262 | −0.676 | −0.580 | −0.458 | −0.587 | −0.573 | −0.099 | 0.850 | 0.842 | 0.752 | 0.859 | 0.896 | 0.864 | 1 |
| Variables | Height | Leaf Area | Fresh Weight | DM% | β Carotene | Lutein+Zea Xanthin | Ascorbic Acid | Chicoric Acid | Rosmarinic Acid | DPPH | ABTS | Phenols | FRAP | P | Ca | Fe | K | Mg | Mn | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Height | 1 | 0.418 | 0.666 | −0.039 | 0.230 | 0.275 | 0.279 | −0.600 | 0.299 | 0.594 | 0.049 | −0.079 | 0.212 | −0.660 | −0.321 | −0.163 | 0.144 | −0.357 | −0.513 | −0.410 |
| Leaf area | 0.418 | 1 | 0.848 | −0.173 | 0.317 | 0.260 | 0.316 | −0.035 | 0.042 | 0.250 | 0.103 | −0.206 | 0.079 | −0.666 | −0.412 | −0.353 | −0.246 | −0.480 | −0.588 | −0.702 |
| Fresh weight | 0.666 | 0.848 | 1 | −0.230 | 0.512 | 0.483 | 0.467 | −0.287 | 0.019 | 0.342 | −0.004 | −0.354 | −0.051 | −0.746 | −0.480 | −0.355 | −0.122 | −0.545 | −0.684 | −0.768 |
| DM% | −0.039 | −0.173 | −0.230 | 1 | −0.706 | −0.739 | −0.692 | −0.246 | 0.322 | 0.354 | 0.646 | 0.695 | 0.606 | −0.230 | −0.143 | 0.077 | 0.043 | 0.023 | −0.106 | 0.069 |
| β carotene | 0.230 | 0.317 | 0.512 | −0.706 | 1 | 0.951 | 0.744 | 0.079 | −0.153 | 0.027 | −0.585 | −0.784 | −0.473 | 0.018 | 0.318 | 0.191 | 0.264 | 0.146 | 0.170 | −0.094 |
| Lutein + Zeaxanthin | 0.275 | 0.260 | 0.483 | −0.739 | 0.951 | 1 | 0.767 | −0.046 | −0.259 | −0.025 | −0.721 | −0.844 | −0.566 | 0.118 | 0.240 | 0.162 | 0.327 | 0.106 | 0.155 | −0.032 |
| Ascorbic acid | 0.279 | 0.316 | 0.467 | −0.692 | 0.744 | 0.767 | 1 | −0.077 | −0.521 | −0.189 | −0.554 | −0.584 | −0.539 | 0.077 | −0.016 | −0.086 | 0.033 | −0.178 | −0.011 | −0.121 |
| Chicoric acid | −0.600 | −0.035 | −0.287 | −0.246 | 0.079 | −0.046 | −0.077 | 1 | −0.285 | −0.651 | −0.036 | 0.040 | −0.271 | 0.354 | 0.233 | −0.197 | −0.515 | 0.115 | 0.321 | −0.001 |
| Rosmarinic acid | 0.299 | 0.042 | 0.019 | 0.322 | −0.153 | −0.259 | −0.521 | −0.285 | 1 | 0.821 | 0.438 | 0.286 | 0.800 | −0.471 | 0.270 | 0.374 | 0.348 | 0.256 | −0.058 | −0.011 |
| DPPH | 0.594 | 0.250 | 0.342 | 0.354 | 0.027 | −0.025 | −0.189 | −0.651 | 0.821 | 1 | 0.344 | 0.108 | 0.713 | −0.619 | 0.093 | 0.391 | 0.514 | 0.143 | −0.208 | −0.085 |
| ABTS | 0.049 | 0.103 | −0.004 | 0.646 | −0.585 | −0.721 | −0.554 | −0.036 | 0.438 | 0.344 | 1 | 0.638 | 0.746 | −0.550 | −0.265 | −0.234 | −0.261 | −0.272 | −0.406 | −0.397 |
| Phenols | −0.079 | −0.206 | −0.354 | 0.695 | −0.784 | −0.844 | −0.584 | 0.040 | 0.286 | 0.108 | 0.638 | 1 | 0.550 | −0.142 | −0.115 | −0.051 | −0.223 | −0.036 | −0.087 | 0.037 |
| FRAP | 0.212 | 0.079 | −0.051 | 0.606 | −0.473 | −0.566 | −0.539 | −0.271 | 0.800 | 0.713 | 0.746 | 0.550 | 1 | −0.502 | 0.025 | 0.135 | 0.108 | 0.000 | −0.209 | −0.115 |
| P | −0.660 | −0.666 | −0.746 | −0.230 | 0.018 | 0.118 | 0.077 | 0.354 | −0.471 | −0.619 | −0.550 | −0.142 | −0.502 | 1 | 0.569 | 0.391 | 0.255 | 0.579 | 0.828 | 0.801 |
| Ca | −0.321 | −0.412 | −0.480 | −0.143 | 0.318 | 0.240 | −0.016 | 0.233 | 0.270 | 0.093 | −0.265 | −0.115 | 0.025 | 0.569 | 1 | 0.835 | 0.659 | 0.905 | 0.906 | 0.735 |
| Fe | −0.163 | −0.353 | −0.355 | 0.077 | 0.191 | 0.162 | −0.086 | −0.197 | 0.374 | 0.391 | −0.234 | −0.051 | 0.135 | 0.391 | 0.835 | 1 | 0.869 | 0.923 | 0.754 | 0.761 |
| K | 0.144 | −0.246 | −0.122 | 0.043 | 0.264 | 0.327 | 0.033 | −0.515 | 0.348 | 0.514 | −0.261 | −0.223 | 0.108 | 0.255 | 0.659 | 0.869 | 1 | 0.720 | 0.537 | 0.609 |
| Mg | −0.357 | −0.480 | −0.545 | 0.023 | 0.146 | 0.106 | −0.178 | 0.115 | 0.256 | 0.143 | −0.272 | −0.036 | 0.000 | 0.579 | 0.905 | 0.923 | 0.720 | 1 | 0.897 | 0.848 |
| Mn | −0.513 | −0.588 | −0.684 | −0.106 | 0.170 | 0.155 | −0.011 | 0.321 | −0.058 | −0.208 | −0.406 | −0.087 | −0.209 | 0.828 | 0.906 | 0.754 | 0.537 | 0.897 | 1 | 0.894 |
| Zn | −0.410 | −0.702 | −0.768 | 0.069 | −0.094 | −0.032 | −0.121 | −0.001 | −0.011 | −0.085 | −0.397 | 0.037 | −0.115 | 0.801 | 0.735 | 0.761 | 0.609 | 0.848 | 0.894 | 1 |
References
- SharathKumar, M.; Heuvelink, E.; Marcelis, L.F.M. Vertical Farming: Moving from Genetic to Environmental Modification. Trends Plant Sci. 2020, 25, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Beacham, A.M.; Vickers, L.H.; Monaghan, J.M. Vertical Farming: A Summary of Approaches to Growing Skywards. J. Hortic. Sci. Biotechnol. 2019, 94, 277–283. [Google Scholar] [CrossRef]
- Avgoustaki, D.D.; Li, J.; Xydis, G. Basil Plants Grown under Intermittent Light Stress in a Small-Scale Indoor Environment: Introducing Energy Demand Reduction Intelligent Technologies. Food Control 2020, 118, 107389. [Google Scholar] [CrossRef]
- Folta, K.M.; Klee, H.J. Sensory Sacrifices When We Mass-Produce Mass Produce. Hortic. Res. 2016, 3, 16032. [Google Scholar] [CrossRef]
- Johnson, A.J.; Meyerson, E.; De La Parra, J.; Savas, T.L.; Miikkulainen, R.; Harper, C.B. Flavor-Cyber-Agriculture: Optimization of Plant Metabolites in an Open-Source Control Environment through Surrogate Modeling. PLoS ONE 2019, 14, e0213918. [Google Scholar] [CrossRef]
- Walters, K.J.; Behe, B.K.; Currey, C.J.; Lopez, R.G. Historical, Current, and Future Perspectives for Controlled Environment Hydroponic Food Crop Production in the United States. Horts 2020, 55, 758–767. [Google Scholar] [CrossRef]
- Huang, L.C. Consumer Attitude, Concerns, and Brand Acceptance for the Vegetables Cultivated with Sustainable Plant Factory Production Systems. Sustainability 2019, 11, 4862. [Google Scholar] [CrossRef]
- Pattison, P.M.; Tsao, J.Y.; Brainard, G.C.; Bugbee, B. LEDs for Photons, Physiology and Food. Nature 2018, 563, 493–500. [Google Scholar] [CrossRef]
- Wong, C.E.; Teo, Z.W.N.; Shen, L.; Yu, H. Seeing the Lights for Leafy Greens in Indoor Vertical Farming. Trends Food Sci. Technol. 2020, 106, 48–63. [Google Scholar] [CrossRef]
- Annunziata, M.G.; Apelt, F.; Carillo, P.; Krause, U.; Feil, R.; Mengin, V.; Lauxmann, M.A.; Köhl, K.; Nikoloski, Z.; Stitt, M.; et al. Getting Back to Nature: A Reality Check for Experiments in Controlled Environments. J. Exp. Bot. 2017, 68, 4463–4477. [Google Scholar] [CrossRef]
- Viršilė, A.; Samuolienė, G.; Miliauskienė, J.; Duchovskis, P. Applications and Advances in LEDs for Horticulture and Crop Production. In Ultraviolet LED Technology for Food Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 35–65. ISBN 9780128177945. [Google Scholar]
- Pennisi, G.; Pistillo, A.; Orsini, F.; Cellini, A.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Crepaldi, A.; Gianquinto, G.; Marcelis, L.F.M. Optimal Light Intensity for Sustainable Water and Energy Use in Indoor Cultivation of Lettuce and Basil under Red and Blue LEDs. Sci. Hortic. 2020, 272, 109508. [Google Scholar] [CrossRef]
- Ng, C.K.-Y. Plant Cell Biology: UVA on Guard. Curr. Biol. 2019, 29, R740–R742. [Google Scholar] [CrossRef]
- Viršilė, A.; Brazaitytė, A.; Vaštakaitė-Kairienė, V.; Miliauskienė, J.; Jankauskienė, J.; Novičkovas, A.; Laužikė, K.; Samuolienė, G. The Distinct Impact of Multi-Color LED Light on Nitrate, Amino Acid, Soluble Sugar and Organic Acid Contents in Red and Green Leaf Lettuce Cultivated in Controlled Environment. Food Chem. 2020, 310, 125799. [Google Scholar] [CrossRef] [PubMed]
- Wargent, J.J. UV LEDs in Horticulture: From Biology to Application. Acta Hortic. 2016, 1134, 25–32. [Google Scholar] [CrossRef]
- Neugart, S.; Schreiner, M. UVB and UVA as Eustressors in Horticultural and Agricultural Crops. Sci. Hortic. 2018, 234, 370–381. [Google Scholar] [CrossRef]
- Chen, Y.; Li, T.; Yang, Q.; Zhang, Y.; Zou, J.; Bian, Z.; Wen, X. UVA Radiation Is Beneficial for Yield and Quality of Indoor Cultivated Lettuce. Front. Plant Sci. 2019, 10, 1563. [Google Scholar] [CrossRef] [PubMed]
- Brazaitytė, A.; Viršilė, A.; Jankauskienė, J.; Sakalauskienė, S.; Samuolienė, G.; Sirtautas, R.; Novičkovas, A.; Dabašinskas, L.; Miliauskienė, J.; Vaštakaitė, V.; et al. Effect of Supplemental UV-A Irradiation in Solid-State Lighting on the Growth and Phytochemical Content of Microgreens. Int. Agrophysics 2015, 29, 13–22. [Google Scholar] [CrossRef]
- Mickens, M.A.; Torralba, M.; Robinson, S.A.; Spencer, L.E.; Romeyn, M.W.; Massa, G.D.; Wheeler, R.M. Growth of Red Pak Choi under Red and Blue, Supplemented White, and Artificial Sunlight Provided by LEDs. Sci. Hortic. 2019, 245, 200–209. [Google Scholar] [CrossRef]
- Samuoliene, G.; Virsile, A.; Miliauskienė, J.; Haimi, P.; Laužikė, K.; Jankauskienė, J.; Novičkovas, A.; Kupčinskienė, A.; Brazaitytė, A. The Photosynthetic Performance of Red Leaf Lettuce under UV-A Irradiation. Agronomy 2020, 10, 761. [Google Scholar] [CrossRef]
- Jensen, N.B.; Clausen, M.R.; Kjaer, K.H. Spectral Quality of Supplemental LED Grow Light Permanently Alters Stomatal Functioning and Chilling Tolerance in Basil (Ocimum basilicum L.). Sci. Hortic. 2018, 227, 38–47. [Google Scholar] [CrossRef]
- Goto, E.; Hayashi, K.; Furuyama, S.; Hikosaka, S.; Ishigami, Y. Effect of UV Light on Phytochemical Accumulation and Expression of Anthocyanin Biosynthesis Genes in Red Leaf Lettuce. Acta Hortic. 2016, 1134, 179–186. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Viršilė, A.; Samuolienė, G.; Vaštakaitė-Kairienė, V.; Jankauskienė, J.; Miliauskienė, J.; Novičkovas, A.; Duchovskis, P. Response of Mustard Microgreens to Different Wavelengths and Durations of UV-A LEDs. Front. Plant Sci. 2019, 10, 1153. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M. Pre-Harvest UV-B Radiation and Photosynthetic Photon Flux Density Interactively Affect Plant Photosynthesis, Growth, and Secondary Metabolites Accumulation in Basil (Ocimum basilicum) Plants. Agronomy 2019, 9, 434. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Ozdemir, F.A.; Kołodziej, B. In Vitro Bioaccessibility and Activity of Basil (Ocimum basilicum L.) Phytochemicals as Affected by Cultivar and Postharvest Preservation Method–Convection Drying, Freezing, and Freeze-Drying. Food Chem. 2022, 382, 132363. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P.; Bhat, T.K. DPPH Antioxidant Assay Revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kraujalytė, V.; Venskutonis, P.R.; Pukalskas, A.; Česonienė, L.; Daubaras, R. Antioxidant Properties and Polyphenolic Compositions of Fruits from Different European Cranberrybush (Viburnum opulus L.) Genotypes. Food Chem. 2013, 141, 3695–3702. [Google Scholar] [CrossRef]
- Romero Rodriguez, M.A.; Vazquez Oderiz, M.L.; Lopez Hernandez, J.; Lozano, J.S. Determination of Vitamin C and Organic Acids in Various Fruits by HPLC. J. Chromatogr. Sci. 1992, 30, 433–437. [Google Scholar] [CrossRef]
- Araújo, G.C.L.; Gonzalez, M.H.; Ferreira, A.G.; Nogueira, A.R.A.; Nóbrega, J.A. Effect of Acid Concentration on Closed-Vessel Microwave-Assisted Digestion of Plant Materials. Spectrochim. Acta Part B At. Spectrosc. 2002, 57, 2121–2132. [Google Scholar] [CrossRef]
- Barbosa, J.T.P.; Santos, C.M.M.; Peralva, V.N.; Flores, E.M.M.; Korn, M.; Nóbrega, J.A.; Korn, M.G.A. Microwave-Assisted Diluted Acid Digestion for Trace Elements Analysis of Edible Soybean Products. Food Chem. 2015, 175, 212–217. [Google Scholar] [CrossRef]
- Choi, D.-S.; Nguyen, T.K.L.; Oh, M.-M. Growth and Biochemical Responses of Kale to Supplementary Irradiation with Different Peak Wavelengths of UV-A Light-Emitting Diodes. Hortic. Environ. Biotechnol. 2022, 63, 65–76. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Viršilė, A.; Samuolienė, G.; Vaštakaitė-Kairienė, V.; Miliauskienė, J.; Jankauskienė, J.; Duchovskis, P. Effect of Supplemental UV-A Radiation on Mineral Elements Content and Nitrate Variation in Brassica Microgreens. Acta Hortic. 2020, 1296, 675–682. [Google Scholar] [CrossRef]
- Appolloni, E.; Pennisi, G.; Zauli, I.; Carotti, L.; Paucek, I.; Quaini, S.; Orsini, F.; Gianquinto, G. Beyond Vegetables: Effects of Indoor LED Light on Specialized Metabolite Biosynthesis in Medicinal and Aromatic Plants, Edible Flowers, and Microgreens. J. Sci. Food Agric. 2022, 102, 472–487. [Google Scholar] [CrossRef]
- Mao, P.; Duan, F.; Zheng, Y.; Yang, Q. Blue and UV-A Light Wavelengths Positively Affected Accumulation Profiles of Healthy Compounds in Pak-choi. J. Sci. Food Agric. 2021, 101, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Zare Mehrjerdi, M.; Aliniaeifard, S.; Seif, M. Photosynthetic and Growth Responses of Green and Purple Basil Plants under Different Spectral Compositions. Physiol. Mol. Biol. Plants 2019, 25, 741–752. [Google Scholar] [CrossRef]
- He, R.; Zhang, Y.; Song, S.; Su, W.; Hao, Y.; Liu, H. UV-A and FR Irradiation Improves Growth and Nutritional Properties of Lettuce Grown in an Artificial Light Plant Factory. Food Chem. 2021, 345, 128727. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Oh, M.-M.; Son, K.-H. Short-Term Ultraviolet (UV)-A Light-Emitting Diode (LED) Radiation Improves Biomass and Bioactive Compounds of Kale. Front. Plant Sci. 2019, 10, 1042. [Google Scholar] [CrossRef]
- Iwai, M.; Ohta, M.; Tsuchiya, H.; Suzuki, T. Enhanced Accumulation of Caffeic Acid, Rosmarinic Acid and Luteolin-Glucoside in Red Perilla Cultivated under Red Diode Laser and Blue LED Illumination Followed by UV-A Irradiation. J. Funct. Foods 2010, 2, 66–70. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of Supplemental Light Quality on Growth and Phytochemicals of Baby Leaf Lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Badmus, U.O.; Ač, A.; Klem, K.; Urban, O.; Jansen, M.A.K. A Meta-Analysis of the Effects of UV Radiation on the Plant Carotenoid Pool. Plant Physiol. Biochem. 2022, 183, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-H.; Huang, M.-Y.; Hsu, M.-H. Morphological and Physiological Response in Green and Purple Basil Plants (Ocimum Basilicum) under Different Proportions of Red, Green, and Blue LED Lightings. Sci. Hortic. 2021, 275, 109677. [Google Scholar] [CrossRef]
- Verdaguer, D.; Jansen, M.A.K.; Llorens, L.; Morales, L.O.; Neugart, S. UV-A Radiation Effects on Higher Plants: Exploring the Known Unknown. Plant Sci. 2017, 255, 72–81. [Google Scholar] [CrossRef] [PubMed]




| DPPH, μmol g−1 | ABTS, μmol g−1 | FRAP, mmol Trolox g−1 | |
|---|---|---|---|
| ‘Italiano classico’ | |||
| RB | 388.0 c | 1113 b | 145.2 a |
| RB + 343 | 416.2 b | 1521 a | 145.7 a |
| RB + 366 | 406.4 b | 1363 ab | 145.7 a |
| RB + 386 | 405.7 b | 1096 b | 138.1 a |
| RB + 402 | 473.2 a | 1197 b | 152.3 a |
| ‘Red rubin’ | |||
| RB | 375.8 b | 1503 b | 141.4 c |
| RB + 343 | 506.6 a | 1619 ab | 157.3 bc |
| RB + 366 | 522.8 a | 1996 a | 183.9 ab |
| RB + 386 | 495.32 a | 2058 a | 189.7 a |
| RB + 402 | 378.8 b | 1881 ab | 154.5 c |
| ‘Italiano Classico’ | |||||||
| mg g−1 DW: | P | Ca | Fe | K | Mg | Mn | Zn |
| RB | 10.54 a | 26.62 a | 0.09 a | 30.24 a | 8.18 a | 0.05 a | 0.04 a |
| RB + 343 | 5.33 d | 14.64 e | 0.06 b | 22.30 c | 5.23 d | 0.03 b | 0.02 c |
| RB + 366 | 6.62 c | 18.43 c | 0.06 b | 25.05 b | 6.32 c | 0.04 ab | 0.03 b |
| RB + 386 | 7.97 b | 22.63 b | 0.09 a | 28.34 a | 7.22 b | 0.04 ab | 0.03 b |
| RB + 402 | 7.15bc | 17.28 d | 0.06 b | 22.63 c | 5.51 cd | 0.04 ab | 0.02 c |
| ‘Red Rubin’ | |||||||
| mg g−1 DW: | P | Ca | Fe | K | Mg | Mn | Zn |
| RB | 15.40 a | 27.37 a | 0.10 a | 29.58 a | 9.58 a | 0.07 a | 0.05 a |
| RB + 343 | 9.46 bc | 25.54 b | 0.10 a | 29.56 a | 9.03 abc | 0.06 b | 0.03 b |
| RB + 366 | 9.33 c | 22.34 c | 0.09 a | 29.16 a | 8.68 bc | 0.05 b | 0.04 ab |
| RB + 386 | 11.29 b | 28.39 a | 0.11 a | 29.05 a | 9.83 a | 0.07 a | 0.04 ab |
| RB + 402 | 10.30 bc | 21.31 c | 0.07 b | 25.33 b | 8.17 c | 0.05 b | 0.03 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viršilė, A.; Laužikė, K.; Sutulienė, R.; Brazaitytė, A.; Kudirka, G.; Samuolienė, G. Distinct Impacts of UV-A Light Wavelengths on Nutraceutical and Mineral Contents in Green and Purple Basil Cultivated in a Controlled Environment. Horticulturae 2023, 9, 1168. https://doi.org/10.3390/horticulturae9111168
Viršilė A, Laužikė K, Sutulienė R, Brazaitytė A, Kudirka G, Samuolienė G. Distinct Impacts of UV-A Light Wavelengths on Nutraceutical and Mineral Contents in Green and Purple Basil Cultivated in a Controlled Environment. Horticulturae. 2023; 9(11):1168. https://doi.org/10.3390/horticulturae9111168
Chicago/Turabian StyleViršilė, Akvilė, Kristina Laužikė, Rūta Sutulienė, Aušra Brazaitytė, Gediminas Kudirka, and Giedrė Samuolienė. 2023. "Distinct Impacts of UV-A Light Wavelengths on Nutraceutical and Mineral Contents in Green and Purple Basil Cultivated in a Controlled Environment" Horticulturae 9, no. 11: 1168. https://doi.org/10.3390/horticulturae9111168