Fermentation of Dietary Fibre-Added Milk with Yoghurt Bacteria and L. rhamnosus and Use in Ice Cream Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
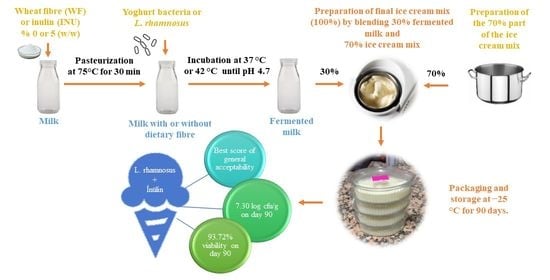
2.2. Preparation of Fermented Milk
2.3. Preparation of Yoghurt and Probiotic Ice Creams
2.4. Determining the Quality Properties of Yoghurt Ice Creams
2.4.1. Viable Cell Counts of Yoghurt and Probiotic Bacteria
2.4.2. Sensory Evaluation
2.4.3. Analysis of Physical Properties
2.4.4. Analysis of Chemical Properties
2.4.5. Analysis of Thermal Properties
2.4.6. Energy (Calorie) Content
2.4.7. Statistical Analysis
3. Results and Discussion
3.1. Viable Cell Counts
3.2. Sensory Evaluation
3.3. Physical and Chemical Properties
3.4. Thermal Properties
3.5. Energy (Calorie) Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al, M.; Ersöz, F.; Özaktaş, T.; Türkanoğlu-Özçelik, A.; Küçükçetin, A. Comparison of the effects of adding microbial transglutaminase to milk and ice cream mixture on the properties of ice cream. Int. J. Dairy Technol. 2020, 73, 578–584. [Google Scholar] [CrossRef]
- Monteiro, S.S.; de Oliveira, V.M.; Pasquali, M.A.d.B. Probiotics in Citrus Fruits Products: Health Benefits and Future Trends for the Production of Functional Foods—A Bibliometric Review. Foods 2022, 11, 1299. [Google Scholar] [CrossRef] [PubMed]
- Zagorska, J.; Paeglite, I.; Galoburda, R. Application of lactobionic acid in ice cream production. Int. J. Dairy Technol. 2022, 75, 701–709. [Google Scholar] [CrossRef]
- Akpinar, A.; Saygili, D.; Yerlikaya, O. Production of set-type yoghurt using Enterococcus faecium and Enterococcus durans strains with probiotic potential as starter adjuncts. Int. J. Dairy Technol. 2020, 73, 726–736. [Google Scholar] [CrossRef]
- Molaee Parvarei, M.; Fazeli, M.R.; Mortazavian, A.M.; Sarem Nezhad, S.; Mortazavi, S.A. Comparative effect of probiotic and paraprobiotic addition on rheological and sensory properties of yoghurt. Int. J. Dairy Technol. 2021, 74, 95–106. [Google Scholar] [CrossRef]
- Zendeboodi, F.; Khorshidian, N.; Mortazavian, A.M.; Cruz, A.G. Probiotic: Conceptualization from a new approach. Curr. Opin. Food Sci. 2020, 32, 103–123. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Sanders, M.E. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, T.C.; de Oliveira, L.I.G.; de Souza, R.C.; Magnani, M. Probiotic ice cream: A literature overview of the technological and sensory aspects and health properties. Int. J. Dairy Technol. 2022, 75, 59–76. [Google Scholar] [CrossRef]
- IDFA (International Dairy Foods Associations). Ice Cream Sales & Trends. Available online: https://www.idfa.org/ice-cream-sales-trends (accessed on 29 November 2022).
- Saremnezhad, S.; Zargarchi, S.; Kalantari, Z.N. Calcium fortification of prebiotic ice-cream. LWT Food Sci. Technol. 2020, 120, 108890. [Google Scholar] [CrossRef]
- Akalın, A.S.; Kesenkas, H.; Dinkci, N.; Unal, G.; Ozer, E.; Kınık, O. Enrichment of probiotic ice cream with different dietary fibres: Structural characteristics and culture viability. J. Dairy Sci. 2018, 101, 37–46. [Google Scholar] [CrossRef]
- Di Criscio, T.; Fratianni, A.; Mignogna, R.; Cinquanta, L.; Coppola, R.; Sorrentino, E.; Panfili, G. Production of functional probiotic, prebiotic, and synbiotic ice creams. J. Dairy Sci. 2010, 93, 4555–4564. [Google Scholar] [CrossRef] [PubMed]
- Akın, M.B.; Akın, M.S.; Kirmacı, Z. Effects of inulin and sugar levels on the viability of yoghurt and probiotic bacteria and the physical and sensory characteristics in probiotic ice-cream. Food Chem. 2007, 104, 93–99. [Google Scholar] [CrossRef]
- Hashemi, M.; Gheisari, H.R.; Shekarforoush, S.S. Survival of Lactobacillus acidophilus and Bacillus coagulans in probiotic and low-fat synbiotic ice-creams. Food Hyg. 2013, 3, 57–65. [Google Scholar]
- Béal, C.; Fonseca, F.; Corrieu, G. Resistance to freezing and frozen storage of Streptococcus thermophilus is related to membrane fatty acid composition. J. Dairy Sci. 2001, 84, 2347–2356. [Google Scholar] [CrossRef] [PubMed]
- Urshev, Z.; Ninova-Nikolova, N.; Ishlimova, D.; Pashova-Baltova, K.; Michaylova, M.; Savova, T. Selection and characterisation of naturally occurring high acidification rate Streptococcus thermophilus strains. Biotechnol. Biotechnol. Equip. 2014, 28, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Rul, F. Yogurt microbiology, organoleptic properties and probiotic potential. In Fermented Foods, 1st ed.; Ray, R.C., Montet, D., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 418–450. [Google Scholar]
- Al-Sulbi, O.S.; Shori, A.B. Viability of selected strains of probiotic Lactobacillus spp. and sensory evaluation of concentrated yogurt (labneh) made from cow, camel, and cashew milk. Food Sci. Technol. 2022, 42, e113321. [Google Scholar] [CrossRef]
- Demirok, N.T.; Durak, M.Z.; Arici, M. Probiotic lactobacilli in faeces of breastfed babies. Food Sci. Technol. 2021, 42, e24821. [Google Scholar] [CrossRef]
- De Farias, T.G.S.; Ladislau, H.F.L.; Stamford, T.C.M.; Medeiros, J.A.C.; Soares, B.L.M.; Arnaud, T.M.S.; Stamford, T.L.M. Viabilities of Lactobacillus rhamnosus ASCC 290 and Lactobacillus casei ATCC 334 (in free form or encapsulated with calcium alginate-chitosan) in yellow mombin ice cream. LWT Food Sci. Technol. 2019, 100, 391–396. [Google Scholar] [CrossRef]
- Abghari, A.; Sheikh-Zeinoddin, M.; Soleimanian-Zad, S. Nonfermented ice cream as a carrier for Lactobacillus acidophilus and Lactobacillus rhamnosus. Int. J. Food Sci. Technol. 2011, 46, 84–92. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Silva, H.L.; Esmerino, E.A.; Rocha, R.S.; Moraes, J.; Carmo, M.A.; Cruz, A.G. The addition of inulin and Lactobacillus casei 01 in sheep milk ice cream. Food Chem. 2018, 246, 464–472. [Google Scholar] [CrossRef]
- Gao, J.; Li, X.; Zhang, G.; Sadiq, F.A.; Simal-Gandara, J.; Xiao, J.; Sang, Y. Probiotics in the dairy industry—Advances and opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3937–3982. [Google Scholar] [CrossRef]
- Alander, M.; Korpela, R.; Saxelin, M.; Vilpponen-Salmela, T.; Mattila-Sandholm, T.; Von Wright, A. Recovery of Lactobacillus rhamnosus GG from human colonic biopsies. Lett. Appl. Microbiol. 1997, 24, 361–364. [Google Scholar] [CrossRef] [PubMed]
- ISO 7889: 2003 (IDF 117: 2003); Yoghurt: Enumeration of Characteristic Microorganisms: Colony-Count Technique at 37 degrees C. International Organization for Standardization: Geneva, Switzerland, 2003.
- Stone, H.; Bleibaum, R.N.; Thomas, H.A. Affective Testing. In Sensory Evaluation Practices, 4th ed.; Stone, H., Bleibaum, R.N., Thomas, H.A., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 291–325. [Google Scholar] [CrossRef]
- Dervisoglu, M.; Yazici, F.; Aydemir, O. The effect of soy protein concentrate addition on the physical, chemical, and sensory properties of strawberry flavored ice cream. Eur. Food Res. Technol. 2005, 221, 466–470. [Google Scholar] [CrossRef]
- Ayar, A.; Siçramaz, H.; Öztürk, S.; Öztürk Yilmaz, S. Probiotic properties of ice creams produced with dietary fibres from by-products of the food industry. Int. J. Dairy Technol. 2018, 71, 174–182. [Google Scholar] [CrossRef]
- Cottrell, J.I.; Pass, G.; Phillips, G.O. Assessment of polysaccharides as ice cream stabilisers. J. Sci. Food and Agric. 1979, 30, 1085–1088. [Google Scholar] [CrossRef]
- Ilansuriyan, P.; Shanmugam, M. Rheological, physiochemical and sensory properties of no fat to high fat ice creams samples prepared using stabiliser/emulsifier blends created with liquid and powder polysorbate-80. Int. Food Res. J. 2018, 25, 2579–2584. [Google Scholar]
- Rossa, P.N.; Burin, V.M.; Bordignon-Luiz, M.T. Effect of microbial transglutaminase on functional and rheological properties of ice cream with different fat contents. LWT Food Sci. Technol. 2012, 48, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC: Washington, DC, USA, 2019; Available online: https://www.aoac.org/official-methods-of-analysis-21st-edition-2019/ (accessed on 29 November 2022).
- Kavaz Yuksel, A. The effects of Blackthorn (Prunus Spinosa L.) addition on certain quality characteristics of ice cream. J. Food Qual. 2015, 38, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Goff, H.D.; Hartel, R.W. Composition and formulations. In Ice Cream, 2nd ed.; Goff, H.D., Hartel, R.W., Eds.; Springer: Boston, MA, USA, 2013; pp. 19–44. [Google Scholar] [CrossRef]
- Charalampopoulos, D.; Pandiella, S.S.; Webb, C. Evaluation of the effect of malt, wheat and barley extracts on the viability of potentially probiotic lactic acid bacteria under acidic conditions. Int. J. Food Microbiol. 2003, 82, 133–141. [Google Scholar] [CrossRef]
- Akalın, A.S.; Erişir, D. Effects of inulin and oligofructose on the rheological characteristics and probiotic culture survival in low-fat probiotic ice cream. J. Food Sci. 2008, 73, M184–M188. [Google Scholar] [CrossRef]
- Hagen, M.; Narvhus, J.A. Production of ice cream containing probiotic bacteria. Milchwissenschaft 1999, 54, 265–268. [Google Scholar]
- Davidson, R.H.; Duncan, S.E.; Hackney, C.R.; Eigel, W.N.; Boling, J.W. Probiotic culture survival and implications in fermented frozen yogurt characteristics. J. Dairy Sci. 2000, 83, 666–673. [Google Scholar] [CrossRef]
- Haynes, I.N.; Playne, M.J. Survival of probiotic cultures in low-fat ice-cream. Aust. J. Dairy Technol. 2002, 57, 10–14. [Google Scholar]
- De Simone, C. The unregulated probiotic market. Clin. Gastroenterol. Hepatol. 2019, 17, 809–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kycia, K.; Chlebowska-Śmigiel, A.; Szydłowska, A.; Sokół, E.; Ziarno, M.; Gniewosz, M. Pullulan as a potential enhancer of Lactobacillus and Bifidobacterium viability in synbiotic low fat yoghurt and its sensory quality. LWT Food Sci. Technol. 2020, 128, 109414. [Google Scholar] [CrossRef]
- Buriti, F.C.; da Rocha, J.S.; Assis, E.G.; Saad, S.M. Probiotic potential of Minas fresh cheese prepared with the addition of Lactobacillus paracasei. LWT Food Sci. Technol. 2005, 38, 173–180. [Google Scholar] [CrossRef]
- Guner, A.; Ardıc, M.; Keles, A.; Dogruer, Y. Production of yogurt ice cream at different acidity. Int. J. Food Sci. Technol. 2007, 42, 948–952. [Google Scholar] [CrossRef]
- Undugoda, L.J.S.; Nilmini, A.H.L. Effect of lactic acid microbial ratio of yoghurt starter culture in yoghurt fermentation and reduction of post acidification. J. Food Ind. Microbiol. 2019, 5, 1–6. [Google Scholar]
- Champagne, C.P.; Gardner, N.J.; Roy, D. Challenges in the addition of probiotic cultures to foods. Crit. Rev. Food Sci. Nutr. 2005, 45, 61–84. [Google Scholar] [CrossRef]
- Muse, M.R.; Hartel, R.W. Ice cream structural elements that affect melting rate and hardness. J. Dairy Sci. 2004, 87, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bolliger, S.; Kornbrust, B.; Goff, H.D.; Tharp, B.W.; Windhab, E.J. Influence of emulsifiers on ice cream produced by conventional freezing and low-temperature extrusion processing. Int. Dairy J. 2000, 10, 497–504. [Google Scholar] [CrossRef]
- Hekmat, S.; McMahon, D.J. Survival of Lactobacillus acidophilus and Bifidobacterium Bifidum in ice cream for use as a probiotic food. J. Dairy Sci. 1992, 75, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Deosarkar, S.S.; Khedkar, C.D.; Kalyankar, S.D.; Sarode, A.R. Ice cream: Uses and method of manufacture. In Encyclopedia of Food and Health, 1st ed.; Caballero, B., Finglas, P.M., Toldra, F., Eds.; Elsevier: Waltham, MA, USA; London, UK, 2016; pp. 391–397. [Google Scholar]
- Khan, S.; Rustagi, S.; Choudhary, S.; Pandey, A.; Khan, M.K.; Kumari, A.; Singh, A. Sucralose and maltodextrin-an alternative to low fat sugar free ice-cream. Biosci. Biotechnol. Res. Commun. 2018, 11, 136–143. [Google Scholar] [CrossRef]

| Sample | Fermented Milk | Mix | Day 1 | Day 30 | Day 60 | Day 90 | |
|---|---|---|---|---|---|---|---|
| Y | L. bulgaricus | 8.41 ± 0.06 DE | 7.64 ± 0.05 Ea | 7.21 ± 0.07 Db | 7.09 ± 0.09 Fc | 6.91 ± 0.14 Ed | 6.72 ± 0.10 De |
| Y-WF | 8.56 ± 0.05 C | 7.67 ± 0.03 DEa | 7.21 ± 0.07 Db | 7.11 ± 0.07 EFc | 7.00 ± 0.08 Ed | 6.78 ± 0.11 De | |
| Y-INU | 8.43 ± 0.05 D | 7.41 ± 0.05 Fa | 7.20 ± 0.09 Db | 7.19 ± 0.06 Eb | 7.13 ± 0.08 Db | 6.80 ± 0.11 Dc | |
| Y | S. thermophilus | 8.84 ± 0.06 B | 8.41 ± 0.05 Ba | 8.42 ± 0.11 ABa | 8.33 ± 0.11 Aab | 8.20 ± 0.13 Bb | 8.00 ± 0.11 Bc |
| Y-WF | 8.97 ± 0.06 A | 8.56 ± 0.04 Aa | 8.46 ± 0.09 Aab | 8.41 ± 0.09 Abc | 8.32 ± 0.07 Ac | 8.21 ± 0.13 Ad | |
| Y-INU | 8.88 ± 0.06 B | 8.43 ± 0.05 Ba | 8.32 ± 0.13 Bab | 8.22 ± 0.10 Bbc | 8.17 ± 0.12 Bc | 7.98 ± 0.10 Bd | |
| LR | L. rhamnosus | 8.37 ± 0.09 DE | 7.81 ± 0.08 Ca | 7.59 ± 0.03 Cb | 7.40 ± 0.06 Dc | 7.40 ± 0.09 Cc | 7.31 ± 0.09 Cc |
| LR-WF | 8.34 ± 0.05 E | 7.72 ± 0.06 Da | 7.51 ± 0.09 Cb | 7.51 ± 0.08 Cb | 7.46 ± 0.07 Cbc | 7.39 ± 0.06 Cc | |
| LR-INU | 8.38 ± 0.08 DE | 7.79 ± 0.06 Ca | 7.61 ± 0.10 Cb | 7.39 ± 0.04 Dc | 7.34 ± 0.10 Cc | 7.30 ± 0.09 Cc |
| Sample | Day 1 | Day 30 | Day 60 | Day 90 | |
|---|---|---|---|---|---|
| Y | L. bulgaricus | 94.44 ± 1.33 Ca | 92.85 ± 1.38 Da | 90.42 ± 1.60 Db | 87.92 ± 1.66 Dc |
| Y-WF | 94.05 ± 1.42 Ca | 92.77 ± 1.30 Dab | 91.27 ± 1.44 Db | 88.47 ± 1.81 Dc | |
| Y-INU | 97.19 ± 0.69 Ba | 97.04 ± 1.33 Ba | 96.21 ± 1.48 ABa | 91.81 ± 1.44 Cb | |
| Y | S. thermophilus | 100.13 ± 1.64 Aa | 99.00 ± 1.44 Aab | 97.53 ± 1.72 Ab | 95.11 ± 1.07 ABc |
| Y-WF | 98.81 ± 1.15 ABa | 98.29 ± 1.29 ABab | 97.24 ± 0.52 Abc | 95.95 ± 1.65 Ac | |
| Y-INU | 98.64 ± 1.39 ABa | 97.48 ± 1.35 ABab | 96.84 ± 1.03 Ab | 94.62 ± 0.74 ABc | |
| LR | L. rhamnosus | 97.18 ± 1.65 Ba | 94.76 ± 1.13 Cb | 94.74 ± 1.10 BCb | 93.57 ± 1.92 Bb |
| LR-WF | 97.30 ± 1.19 Ba | 97.31 ± 1.32 Ba | 96.63 ± 0.67 Aab | 95.81 ± 0.77 Ab | |
| LR-INU | 97.66 ± 1.99 Ba | 94.87 ± 1.22 Cb | 94.24 ± 1.99 Cb | 93.72 ± 1.73 Bb |
| Sample | Elongation at Spoon | Iciness | Degree of Sweetness | Degree of Sourness | Melting Time in Mouth | Colour and Appearance | Structure and Consistency | Taste and Smell | General Acceptability |
|---|---|---|---|---|---|---|---|---|---|
| C | 4.81 ± 1.39 A | 1.79 ± 1.89 A | 5.53 ± 0.84 A | 2.94 ± 2.02 A | 5.02 ± 1.09 A | 7.93 ± 0.97 A | 7.22 ± 1.03 AB | 7.38 ± 1.03 AB | 7.33 ± 1.04 ABC |
| Y | 4.44 ± 1.78 A | 1.67 ± 0.88 A | 5.19 ± 1.00 A | 4.15 ± 2.20 A | 5.30 ± 1.14 A | 8.00 ± 0.88 A | 7.56 ± 1.05 A | 7.44 ± 1.01 AB | 7.44 ± 1.05 AB |
| Y-WF | 4.11 ± 1.69 A | 1.89 ± 1.12 A | 5.07 ± 0.92 A | 4.15 ± 2.23 A | 5.19 ± 1.21 A | 7.85 ± 1.13 A | 6.74 ± 1.63 B | 6.44 ± 1.22 C | 6.78 ± 1.19 C |
| Y-INU | 4.74 ± 1.70 A | 1.56 ± 0.89 A | 5.44 ± 1.01 A | 3.85 ± 2.11 A | 5.22 ± 1.01 A | 7.93 ± 1.04 A | 7.41 ± 1.15 AB | 7.04 ± 1.19 B | 7.15 ± 1.06 BC |
| LR | 4.19 ± 1.75 A | 1.74 ± 0.94 A | 5.15 ± 1.17 A | 3.19 ± 2.15 A | 5.04 ± 1.29 A | 8.07 ± 0.87 A | 7.22 ± 1.19 AB | 7.59 ± 1.05 AB | 7.70 ± 0.78 AB |
| LR-WF | 4.37 ± 1.67 A | 1.33 ± 0.78 A | 5.44 ± 1.12 A | 3.89 ± 2.22 A | 5.44 ± 1.12 A | 7.81 ± 1.08 A | 6.81 ± 1.78 AB | 7.22 ± 1.12 B | 7.30 ± 1.17 ABC |
| LR-INU | 4.85 ± 1.92 A | 1.93 ± 1.00 A | 5.44 ± 1.25 A | 3.30 ± 2.20 A | 5.19 ± 1.39 A | 8.11 ± 0.70 A | 7.56 ± 1.15 A | 7.93 ± 0.78 A | 7.81 ± 0.79 A |
| Properties | C | Y | Y-WF | Y-INU | LR | LR-WF | LR-INU |
|---|---|---|---|---|---|---|---|
| Viscosity (Pa.s) | 1.08 ± 0.07 d | 2.28 ± 0.06 c | 2.77 ± 0.03 a | 2.69 ± 0.05 a | 2.30 ± 0.09 c | 2.57 ± 0.09 b | 2.53 ± 0.10 b |
| Overrun (%) | 22.6 ± 1.8 a | 22.9 ± 2.0 a | 15.5 ± 2.2 bc | 17.9 ± 2.8 b | 22.8 ± 2.9 a | 13.6 ± 3.8 c | 16.1 ± 2.3 bc |
| First dripping (sec) | 1998 ± 34 b | 2053 ± 43 b | 2183 ± 49 a | 1992 ± 44 b | 2007 ± 48 b | 2213 ± 42 a | 2018 ± 70 b |
| Complete melting (sec) | 3311 ± 55 a | 3078 ± 30 c | 3017 ± 65 cd | 2987 ± 43 d | 3259 ± 70 ab | 3326 ± 79 a | 3225 ± 71 b |
| Hardness (N) | 1.20 ± 0.11 c | 1.53 ± 0.10 b | 1.78 ± 0.17 a | 1.49 ± 0.15 b | 1.22 ± 0.11 c | 1.44 ± 0.12 b | 1.23 ± 0.09 c |
| Fat destabilisation (%) | 12.76 ± 0.55 f | 21.81 ± 0.85 b | 17.49 ± 0.48 d | 10.39 ± 0.55 g | 31.43 ± 1.17 a | 20.19 ± 0.87 c | 14.89 ± 0.47 e |
| pH | 6.56 ± 0.02 a | 6.24 ± 0.02 cd | 6.23 ± 0.02 d | 6.23 ± 0.03 d | 6.25 ± 0.03 bcd | 6.28 ± 0.02 b | 6.27 ± 0.02 bc |
| Lactic acid (%) | 0.21 ± 0.02 c | 0.37 ± 0.01 a | 0.38 ± 0.02 a | 0.37 ± 0.01 a | 0.33 ± 0.01 b | 0.33 ± 0.01 b | 0.32 ± 0.01 b |
| Total solids (%) | 35.09 ± 0.16 e | 35.14 ± 0.14 e | 36.20 ± 0.19 c | 36.70 ± 0.10 a | 35.34 ± 0.12 d | 36.65 ± 0.15 ab | 36.50 ± 0.17 b |
| Fat (%) | 5.82 ± 0.26 a | 5.98 ± 0.27 a | 5.92 ± 0.22 a | 5.85 ± 0.16 a | 5.92 ± 0.22 a | 5.97 ± 0.20 a | 5.95 ± 0.20 a |
| Properties | C | Y | Y-WF | Y-INU | LR | LR-WF | LR-INU |
|---|---|---|---|---|---|---|---|
| Tm (°C) | 1.88 ± 0.96 a | 0.79 ± 0.11 bc | −1.15 ± 0.27 e | 0.45 ± 0.31 bcd | 1.20 ± 0.35 ab | −0.09 ± 0.50 d | 0.00 ± 0.05 cd |
| Tg (°C) | −28.65 ± 1.59 a | −28.61 ± 1.04 a | −30.72 ± 1.75 a | −28.31 ± 1.70 a | −28.72 ± 1.04 a | −29.68 ± 1.17 a | −30.09 ± 0.95 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sezer, E.; Ayar, A.; Yılmaz, S.Ö. Fermentation of Dietary Fibre-Added Milk with Yoghurt Bacteria and L. rhamnosus and Use in Ice Cream Production. Fermentation 2023, 9, 3. https://doi.org/10.3390/fermentation9010003
Sezer E, Ayar A, Yılmaz SÖ. Fermentation of Dietary Fibre-Added Milk with Yoghurt Bacteria and L. rhamnosus and Use in Ice Cream Production. Fermentation. 2023; 9(1):3. https://doi.org/10.3390/fermentation9010003
Chicago/Turabian StyleSezer, Elif, Ahmet Ayar, and Suzan Öztürk Yılmaz. 2023. "Fermentation of Dietary Fibre-Added Milk with Yoghurt Bacteria and L. rhamnosus and Use in Ice Cream Production" Fermentation 9, no. 1: 3. https://doi.org/10.3390/fermentation9010003