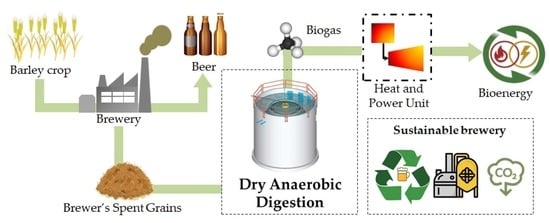
Dry Anaerobic Digestion of Brewer’s Spent Grains toward a More Sustainable Brewery: Operational Performance, Kinetic Analysis, and Bioenergy Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solid Wastes and Inoculum
2.2. Reactor Configuration for Dry AD
2.3. Physicochemical Analysis
2.4. Biogas Production, Composition, and Yield
2.5. Kinetic Analysis
2.6. Bioenergy Recovery and Avoided GHG Emissions
2.7. Industrial Design of AD for Bioenergy Recovery
2.8. Statistical Analysis
3. Results and Discussion
3.1. Operational Performance of the Dry AD Reactor
3.1.1. pH and Alkalinity
3.1.2. Soluble Chemical Oxygen Demand
3.1.3. Ammonia Nitrogen
3.1.4. Solids
3.2. Biogas Production and Composition
3.3. Kinetic Analysis
3.4. Recovery of Bioenergy from Biogas Potential and Avoided GHG Emissions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brasil Anuário da Cerveja 2021—Ministério da Agricultura, Pecuária e Abastecimento. 2021. Available online: https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-vegetal/publicacoes/anuario-da-cerveja-2021.pdf (accessed on 10 September 2022).
- Mussatto, S.I. Brewer’s Spent Grain: A Valuable Feedstock for Industrial Applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IBGE Anuário Estatístico do Brasil. Available online: https://biblioteca.ibge.gov.br/visualizacao/periodicos/20/aeb_2019.pdf (accessed on 11 September 2022).
- CONAB Companhia Nacional de Abastecimento—Importações e Exportações. Available online: www.portaldeinformacoes.conab.gov.br/comercio-exterior-por-pais (accessed on 12 September 2022).
- Ricciardi, P.; Cillari, G.; Carnevale Miino, M.; Collivignarelli, M.C. Valorization of Agro-Industry Residues in the Building and Environmental Sector: A Review. Waste Manag. Res. 2020, 38, 487–513. [Google Scholar] [CrossRef] [PubMed]
- González-García, S.; Morales, P.C.; Gullón, B. Estimating the Environmental Impacts of a Brewery Waste–Based Biorefinery: Bio-Ethanol and Xylooligosaccharides Joint Production Case Study. Ind. Crop. Prod. 2018, 123, 331–340. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ Spent Grain: Generation, Characteristics and Potential Applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Torres-Mayanga, P.C.; Azambuja, S.P.H.; Tyufekchiev, M.; Tompsett, G.A.; Timko, M.T.; Goldbeck, R.; Rostagno, M.A.; Forster-Carneiro, T. Subcritical Water Hydrolysis of Brewer’s Spent Grains: Selective Production of Hemicellulosic Sugars (C-5 Sugars). J. Supercrit. Fluids 2019, 145, 19–30. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Buller, L.S.; Mussatto, S.I.; Forster-Carneiro, T. Techno-Economic Assessment of Bioenergy and Fertilizer Production by Anaerobic Digestion of Brewer’s Spent Grains in a Biorefinery Concept. J. Clean. Prod. 2021, 297, 126600. [Google Scholar] [CrossRef]
- Margallo, M.; Ziegler-Rodriguez, K.; Vázquez-Rowe, I.; Aldaco, R.; Irabien, Á.; Kahhat, R. Enhancing Waste Management Strategies in Latin America under a Holistic Environmental Assessment Perspective: A Review for Policy Support. Sci. Total Environ. 2019, 689, 1255–1275. [Google Scholar] [CrossRef]
- Jiménez-Castro, M.P.; Buller, L.S.; Sganzerla, W.G.; Forster-Carneiro, T. Bioenergy Production from Orange Industrial Waste: A Case Study. Biofuels Bioprod. Biorefining 2020, 14, 1239–1253. [Google Scholar] [CrossRef]
- Das, S.; Lee, S.-H.; Kumar, P.; Kim, K.-H.; Lee, S.S.; Bhattacharya, S.S. Solid Waste Management: Scope and the Challenge of Sustainability. J. Clean. Prod. 2019, 228, 658–678. [Google Scholar] [CrossRef]
- Ferreira, S.F.; Buller, L.S.; Maciel-Silva, F.W.; Sganzerla, W.G.; Berni, M.D.; Forster-Carneiro, T. Waste Management and Bioenergy Recovery from Açaí Processing in the Brazilian Amazonian Region: A Perspective for a Circular Economy. Biofuels Bioprod. Biorefining 2021, 15, 37–46. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Ampese, L.C.; Mussatto, S.I.; Forster-Carneiro, T. A Bibliometric Analysis on Potential Uses of Brewer’s Spent Grains in a Biorefinery for the Circular Economy Transition of the Beer Industry. Biofuels Bioprod. Biorefining 2021, 15, bbb.2290. [Google Scholar] [CrossRef]
- Di Domenico Ziero, H.; Ampese, L.C.; Sganzerla, W.G.; Torres-Mayanga, P.C.; Timko, M.T.; Mussatto, S.I.; Forster-Carneiro, T. Subcritical Water Hydrolysis of Poultry Feathers for Amino Acids Production. J. Supercrit. Fluids 2022, 181, 105492. [Google Scholar] [CrossRef]
- Paudel, S.R.; Banjara, S.P.; Choi, O.K.; Park, K.Y.; Kim, Y.M.; Lee, J.W. Pretreatment of Agricultural Biomass for Anaerobic Digestion: Current State and Challenges. Bioresour. Technol. 2017, 245, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Rocamora, I.; Wagland, S.T.; Villa, R.; Simpson, E.W.; Fernández, O.; Bajón-Fernández, Y. Dry Anaerobic Digestion of Organic Waste: A Review of Operational Parameters and Their Impact on Process Performance. Bioresour. Technol. 2020, 299, 122681. [Google Scholar] [CrossRef]
- Bi, S.; Westerholm, M.; Qiao, W.; Xiong, L.; Mahdy, A.; Yin, D.; Song, Y.; Dong, R. Metabolic Performance of Anaerobic Digestion of Chicken Manure under Wet, High Solid, and Dry Conditions. Bioresour. Technol. 2020, 296, 122342. [Google Scholar] [CrossRef]
- Zhang, E.; Li, J.; Zhang, K.; Wang, F.; Yang, H.; Zhi, S.; Liu, G. Anaerobic Digestion Performance of Sweet Potato Vine and Animal Manure under Wet, Semi-Dry, and Dry Conditions. AMB Express 2018, 8, 45. [Google Scholar] [CrossRef]
- Di Maria, F.; Barratta, M.; Bianconi, F.; Placidi, P.; Passeri, D. Solid Anaerobic Digestion Batch with Liquid Digestate Recirculation and Wet Anaerobic Digestion of Organic Waste: Comparison of System Performances and Identification of Microbial Guilds. Waste Manag. 2017, 59, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Shi, J.; Li, Y. Comparison of Solid-State to Liquid Anaerobic Digestion of Lignocellulosic Feedstocks for Biogas Production. Bioresour. Technol. 2012, 124, 379–386. [Google Scholar] [CrossRef]
- Abbassi-Guendouz, A.; Brockmann, D.; Trably, E.; Dumas, C.; Delgenès, J.-P.; Steyer, J.-P.; Escudié, R. Total Solids Content Drives High Solid Anaerobic Digestion via Mass Transfer Limitation. Bioresour. Technol. 2012, 111, 55–61. [Google Scholar] [CrossRef]
- Yang, T.; Li, Y.; Gao, J.; Huang, C.; Chen, B.; Zhang, L.; Wang, X.; Zhao, Y.; Xi, B.; Li, X. Performance of Dry Anaerobic Technology in the Co-Digestion of Rural Organic Solid Wastes in China. Energy 2015, 93, 2497–2502. [Google Scholar] [CrossRef]
- Buller, L.S.; da Silva Romero, C.W.; Lamparelli, R.A.C.; Ferreira, S.F.; Bortoleto, A.P.; Mussatto, S.I.; Forster-Carneiro, T. A Spatially Explicit Assessment of Sugarcane Vinasse as a Sustainable By-Product. Sci. Total Environ. 2021, 765, 142717. [Google Scholar] [CrossRef] [PubMed]
- Rasapoor, M.; Young, B.; Brar, R.; Sarmah, A.; Zhuang, W.-Q.; Baroutian, S. Recognizing the Challenges of Anaerobic Digestion: Critical Steps toward Improving Biogas Generation. Fuel 2020, 261, 116497. [Google Scholar] [CrossRef]
- Kainthola, J.; Kalamdhad, A.S.; Goud, V. V A Review on Enhanced Biogas Production from Anaerobic Digestion of Lignocellulosic Biomass by Different Enhancement Techniques. Process Biochem. 2019, 84, 81–90. [Google Scholar] [CrossRef]
- da Rosa, R.G.; Sganzerla, W.G.; Barroso, T.L.C.T.; Castro, L.E.N.; Berni, M.D.; Forster-Carneiro, T. Sustainable Bioprocess Combining Subcritical Water Pretreatment Followed by Anaerobic Digestion for the Valorization of Jabuticaba (Myrciaria Cauliflora) Agro-Industrial by-Product in Bioenergy and Biofertilizer. Fuel 2023, 334, 126698. [Google Scholar] [CrossRef]
- Sillero, L.; Sganzerla, W.G.; Carneiro, T.F.; Solera, R.; Perez, M. Techno-Economic Analysis of Single-Stage and Temperature-Phase Anaerobic Co-Digestion of Sewage Sludge, Wine Vinasse, and Poultry Manure. J. Environ. Manag. 2023, 325, 116419. [Google Scholar] [CrossRef] [PubMed]
- Sganzerla, W.G.; Ampese, L.C.; Parisoto, T.A.C.; Forster-Carneiro, T. Process Intensification for the Recovery of Methane-Rich Biogas from Dry Anaerobic Digestion of Açaí Seeds. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Keijer, T.; Bakker, V.; Slootweg, J.C. Circular Chemistry to Enable a Circular Economy. Nat. Chem. 2019, 11, 190–195. [Google Scholar] [CrossRef]
- Geng, Y.; Sarkis, J.; Bleischwitz, R. How to Globalize the Circular Economy. Nature 2019, 565, 153–155. [Google Scholar] [CrossRef] [Green Version]
- Hysa, E.; Kruja, A.; Rehman, N.U.; Laurenti, R. Circular Economy Innovation and Environmental Sustainability Impact on Economic Growth: An Integrated Model for Sustainable Development. Sustainability 2020, 12, 4831. [Google Scholar] [CrossRef]
- Weber, C.T.; Trierweiler, L.F.; Trierweiler, J.O. Food Waste Biorefinery Advocating Circular Economy: Bioethanol and Distilled Beverage from Sweet Potato. J. Clean. Prod. 2020, 268, 121788. [Google Scholar] [CrossRef]
- Dragone, G.; Kerssemakers, A.A.J.; Driessen, J.L.S.P.; Yamakawa, C.K.; Brumano, L.P.; Mussatto, S.I. Innovation and Strategic Orientations for the Development of Advanced Biorefineries. Bioresour. Technol. 2020, 302, 122847. [Google Scholar] [CrossRef] [PubMed]
- IEA Bioenergy—Task 42 Biorefining in a Circular Economy. Available online: http://task42.ieabioenergy.com/ (accessed on 15 September 2022).
- Mussatto, S.I.; Moncada, J.; Roberto, I.C.; Cardona, C.A. Techno-Economic Analysis for Brewer’s Spent Grains Use on a Biorefinery Concept: The Brazilian Case. Bioresour. Technol. 2013, 148, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Zhang, T.; Wang, X.; Feng, Y.; Ren, G.; Yang, G. Process Performance and Methane Production Optimizing of Anaerobic Co-Digestion of Swine Manure and Corn Straw. Sci. Rep. 2017, 7, 9379. [Google Scholar] [CrossRef] [Green Version]
- APHA—American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; Rice, E.W., Baird, R.B., Eaton, A.D., Eds.; American Public Health Association, American Water Works Association, and Water Environment Federation: Washington, DC, USA, 2017; ISBN 978-0-87553-287-5. [Google Scholar]
- Sillero, L.; Sganzerla, W.G.; Forster-Carneiro, T.; Solera, R.; Perez, M. A Bibliometric Analysis of the Hydrogen Production from Dark Fermentation. Int. J. Hydrogen Energy 2022, 47, 27397–27420. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Tena-Villares, M.; Buller, L.S.; Mussatto, S.I.; Forster-Carneiro, T. Dry Anaerobic Digestion of Food Industry By-Products and Bioenergy Recovery: A Perspective to Promote the Circular Economy Transition. Waste Biomass Valorization 2022, 13, 2575–2589. [Google Scholar] [CrossRef]
- Campello, L.D.; Barros, R.M.; Tiago Filho, G.L.; dos Santos, I.F.S. Analysis of the Economic Viability of the Use of Biogas Produced in Wastewater Treatment Plants to Generate Electrical Energy. Environ. Dev. Sustain. 2021, 23, 2614–2629. [Google Scholar] [CrossRef]
- CHP Brasil CHP Brasil. Available online: https://chpbrasil.com.br/en/solucoes/cogeracao-qualificada (accessed on 17 September 2022).
- dos Santos, I.F.S.; Braz Vieira, N.D.; de Nóbrega, L.G.B.; Barros, R.M.; Tiago Filho, G.L. Assessment of Potential Biogas Production from Multiple Organic Wastes in Brazil: Impact on Energy Generation, Use, and Emissions Abatement. Resour. Conserv. Recycl. 2018, 131, 54–63. [Google Scholar] [CrossRef]
- MCTIC Ministério da Ciência, Tecnologia, Inovações e Comunicações. Available online: https://www.mctic.gov.br/mctic/opencms/ciencia/SEPED/clima/textogeral/emissao_corporativos.htm (accessed on 17 September 2022).
- IPCC. IPCC Guidelines for National Greenhouse Gas Inventories—Volume 2: Energy. 2006. Available online: https://www.ipcc-nggip.iges.or.jp/public/2006gl/vol2.html (accessed on 19 September 2022).
- Jin, Q.; Kirk, M.F. PH as a Primary Control in Environmental Microbiology: 1. Thermodynamic Perspective. Front. Environ. Sci. 2018, 6, 21. [Google Scholar] [CrossRef]
- Kumar, A.; Samadder, S.R. Performance Evaluation of Anaerobic Digestion Technology for Energy Recovery from Organic Fraction of Municipal Solid Waste: A Review. Energy 2020, 197, 117253. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Surendra, K.C.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic Digestion of Lignocellulosic Biomass: Challenges and Opportunities. Bioresour. Technol. 2015, 178, 178–186. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Wu, J. Enhancement of Methane Production in Anaerobic Digestion Process: A Review. Appl. Energy 2019, 240, 120–137. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Berni, M.; Dragone, G.; Mussatto, S.I.; Forster-Carneiro, T. Anaerobic Digestion Process: Technological Aspects and Recent Developments. Int. J. Environ. Sci. Technol. 2018, 15, 2033–2046. [Google Scholar] [CrossRef]
- Jiménez-Castro, M.P.; Buller, L.S.; Zoffreo, A.; Timko, M.T.; Forster-Carneiro, T. Two-Stage Anaerobic Digestion of Orange Peel without Pre-Treatment: Experimental Evaluation and Application to São Paulo State. J. Environ. Chem. Eng. 2020, 8, 104035. [Google Scholar] [CrossRef]
- Maciel-Silva, F.W.; Mussatto, S.I.; Forster-Carneiro, T. Integration of Subcritical Water Pretreatment and Anaerobic Digestion Technologies for Valorization of Açai Processing Industries Residues. J. Clean. Prod. 2019, 228, 1131–1142. [Google Scholar] [CrossRef]
- Atelge, M.R.; Atabani, A.E.; Banu, J.R.; Krisa, D.; Kaya, M.; Eskicioglu, C.; Kumar, G.; Lee, C.; Yildiz, Y.Ş.; Unalan, S.; et al. A Critical Review of Pretreatment Technologies to Enhance Anaerobic Digestion and Energy Recovery. Fuel 2020, 270, 117494. [Google Scholar] [CrossRef]
- Castro, L.E.N.; Colpini, L.M.S. All-around Characterization of Brewers’ Spent Grain. Eur. Food Res. Technol. 2021, 247, 3013–3021. [Google Scholar] [CrossRef]
- Qin, F.; Johansen, A.Z.; Mussatto, S.I. Evaluation of Different Pretreatment Strategies for Protein Extraction from Brewer’s Spent Grains. Ind. Crop. Prod. 2018, 125, 443–453. [Google Scholar] [CrossRef]
- Wang, X.; Gabauer, W.; Li, Z.; Ortner, M.; Fuchs, W. Improving Exploitation of Chicken Manure via Two-Stage Anaerobic Digestion with an Intermediate Membrane Contactor to Extract Ammonia. Bioresour. Technol. 2018, 268, 811–814. [Google Scholar] [CrossRef]
- Andreoli, C.V.; von Sperling, M.; Fernandes, F. Sludge Treatment and Disposal. Water Intell. Online 2015, 6, 9781780402130. [Google Scholar] [CrossRef]
- Rico, C.; Montes, J.A.; Lobo, A. Dry Batch Anaerobic Digestion of Food Waste in a Box-Type Reactor System: Inoculum Preparation and Reactor Performance. J. Clean. Prod. 2020, 251, 119751. [Google Scholar] [CrossRef]
- Yao, Y.; Luo, Y.; Yang, Y.; Sheng, H.; Li, X.; Li, T.; Song, Y.; Zhang, H.; Chen, S.; He, W.; et al. Water Free Anaerobic Co-Digestion of Vegetable Processing Waste with Cattle Slurry for Methane Production at High Total Solid Content. Energy 2014, 74, 309–313. [Google Scholar] [CrossRef]
- Gonçalves, I.C.; Fonseca, A.; Morão, A.M.; Pinheiro, H.M.; Duarte, A.P.; Ferra, M.I.A. Evaluation of Anaerobic Co-Digestion of Spent Brewery Grains and an Azo Dye. Renew. Energy 2015, 74, 489–496. [Google Scholar] [CrossRef]
- Ampese, L.C.; Sganzerla, W.G.; Di Domenico Ziero, H.; Costa, J.M.; Martins, G.; Forster-Carneiro, T. Valorization of Apple Pomace for Biogas Production: A Leading Anaerobic Biorefinery Approach for a Circular Bioeconomy. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Buller, L.S.; Sganzerla, W.G.; Lima, M.N.; Muenchow, K.E.; Timko, M.T.; Forster-Carneiro, T. Ultrasonic Pretreatment of Brewers’ Spent Grains for Anaerobic Digestion: Biogas Production for a Sustainable Industrial Development. J. Clean. Prod. 2022, 355, 131802. [Google Scholar] [CrossRef]
- Chen, R.; Li, Z.; Feng, J.; Zhao, L.; Yu, J. Effects of Digestate Recirculation Ratios on Biogas Production and Methane Yield of Continuous Dry Anaerobic Digestion. Bioresour. Technol. 2020, 316, 123963. [Google Scholar] [CrossRef] [PubMed]
- Patinvoh, R.J.; Kalantar Mehrjerdi, A.; Sárvári Horváth, I.; Taherzadeh, M.J. Dry Fermentation of Manure with Straw in Continuous Plug Flow Reactor: Reactor Development and Process Stability at Different Loading Rates. Bioresour. Technol. 2017, 224, 197–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veluchamy, C.; Gilroyed, B.H.; Kalamdhad, A.S. Process Performance and Biogas Production Optimizing of Mesophilic Plug Flow Anaerobic Digestion of Corn Silage. Fuel 2019, 253, 1097–1103. [Google Scholar] [CrossRef]
- Tena, M.; Luque, B.; Perez, M.; Solera, R. Enhanced Hydrogen Production from Sewage Sludge by Cofermentation with Wine Vinasse. Int. J. Hydrogen Energy 2020, 45, 15977–15984. [Google Scholar] [CrossRef]
- Tena, M.; Perez, M.; Solera, R. Effects of Several Inocula on the Biochemical Hydrogen Potential of Sludge-Vinasse Co-Digestion. Fuel 2019, 258, 116180. [Google Scholar] [CrossRef]
- Muench, S. Greenhouse Gas Mitigation Potential of Electricity from Biomass. J. Clean. Prod. 2015, 103, 483–490. [Google Scholar] [CrossRef]
- Bertrand, V.; Dequiedt, B.; Le Cadre, E. Biomass for Electricity in the EU-27: Potential Demand, CO2 Abatements and Breakeven Prices for Co-Firing. Energy Policy 2014, 73, 631–644. [Google Scholar] [CrossRef]
- Loução, P.O.; Ribau, J.P.; Ferreira, A.F. Life Cycle and Decision Analysis of Electricity Production from Biomass—Portugal Case Study. Renew. Sustain. Energy Rev. 2019, 108, 452–480. [Google Scholar] [CrossRef]
- Olajire, A.A. The Brewing Industry and Environmental Challenges. J. Clean. Prod. 2020, 256, 102817. [Google Scholar] [CrossRef]
- Rajaniemi, M.; Mikkola, H.; Ahokas, J. Greenhouse Gas Emissions from Oats, Barley, Wheat and Rye Production. Agron. Res. 2011, 9, 189–195. [Google Scholar]
- Carr-Harris, H. Environmental Management in the Brewing Industry; United Nations Environment Programme, Industry and Environment: Paris, France, 1996; ISBN 9789280715231. [Google Scholar]
- Garcia-Garcia, G.; Rahimifard, S. Life-Cycle Environmental Impacts of Barley Straw Valorisation. Resour. Conserv. Recycl. 2019, 149, 1–11. [Google Scholar] [CrossRef]
- Leme, R.M.; Seabra, J.E.A. Technical-Economic Assessment of Different Biogas Upgrading Routes from Vinasse Anaerobic Digestion in the Brazilian Bioethanol Industry. Energy 2017, 119, 754–766. [Google Scholar] [CrossRef]
- Mudhoo, A.; Torres-Mayanga, P.C.; Forster-Carneiro, T.; Sivagurunathan, P.; Kumar, G.; Komilis, D.; Sánchez, A. A Review of Research Trends in the Enhancement of Biomass-to-Hydrogen Conversion. Waste Manag. 2018, 79, 580–594. [Google Scholar] [CrossRef] [Green Version]
- Freitas, F.F.; De Souza, S.S.; Ferreira, L.R.A.; Otto, R.B.; Alessio, F.J.; De Souza, S.N.M.; Venturini, O.J.; Ando Junior, O.H. The Brazilian Market of Distributed Biogas Generation: Overview, Technological Development and Case Study. Renew. Sustain. Energy Rev. 2019, 101, 146–157. [Google Scholar] [CrossRef]
- Garcia, N.H.; Mattioli, A.; Gil, A.; Frison, N.; Battista, F.; Bolzonella, D. Evaluation of the Methane Potential of Different Agricultural and Food Processing Substrates for Improved Biogas Production in Rural Areas. Renew. Sustain. Energy Rev. 2019, 112, 1–10. [Google Scholar] [CrossRef]
- Diniz, D.; Carvalho, M.; Abrahão, R. Greenhouse Gas Accounting for the Energy Transition in a Brewery. Environ. Prog. Sustain. Energy 2021, 40, e13563. [Google Scholar] [CrossRef]
- Fadare, D.A.; Nkpubre, D.O.; Oni, A.O.; Falana, A.; Waheed, M.A.; Bamiro, O.A. Energy and Exergy Analyses of Malt Drink Production in Nigeria. Energy 2010, 35, 5336–5346. [Google Scholar] [CrossRef]
- Collet, P.; Hélias, A.; Lardon, L.; Ras, M.; Goy, R.-A.; Steyer, J.-P. Life-Cycle Assessment of Microalgae Culture Coupled to Biogas Production. Bioresour. Technol. 2011, 102, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Bernstad, A.; la Cour Jansen, J. A Life Cycle Approach to the Management of Household Food Waste—A Swedish Full-Scale Case Study. Waste Manag. 2011, 31, 1879–1896. [Google Scholar] [CrossRef] [PubMed]
- Wainaina, S.; Awasthi, M.K.; Sarsaiya, S.; Chen, H.; Singh, E.; Kumar, A.; Ravindran, B.; Awasthi, S.K.; Liu, T.; Duan, Y.; et al. Resource Recovery and Circular Economy from Organic Solid Waste Using Aerobic and Anaerobic Digestion Technologies. Bioresour. Technol. 2020, 301, 122778. [Google Scholar] [CrossRef]




| Parameters | BSG | Inoculum * | Unit |
|---|---|---|---|
| pH | 6.65 ± 0.01 b | 7.78 ± 0.21 a | – |
| Density | 4108 ± 2.51 a | 1023.16 ± 1.54 b | g L−1 |
| Alkalinity | 707.75 ± 42.75 a | 194.75 ± 4.75 b | mg CaCO3 L−1 |
| Chemical oxygen demand | 1519.04 ± 23.82 a | 15.77 ± 2.00 b | mg O2 L−1 |
| Ammonia nitrogen | 190.54 ± 20.27 a | 23.94 ± 2.66 b | mg N-NH3 L−1 |
| Moisture | 3.49 ± 0.06 b | 89.52 ± 0.56 a | % |
| Total solids | 96.68 ± 0.21 a | 10.48 ± 0.53 b | % |
| Total volatile solids | 73.62 ± 0.58 a | 8.47 ± 0.31 b | % |
| Total fixed solids | 23.06 ± 0.21 a | 2.01 ± 0.08 b | % |
| Parameters | Initial (Day 0) | Final (Day 40) | Unit |
|---|---|---|---|
| pH | 6.11 ± 0.54 | 8.53 ± 0.15 | – |
| Alkalinity | 189.04 ± 15.32 | 717.46 ± 23.38 | mg CaCO3 L−1 |
| COD | 16,239.60 ± 346.84 | 6922.73 ± 142.94 | mg O2 L−1 |
| COD removal | – | 57.38 | % |
| Ammonia nitrogen | 118.36 ± 19.45 | 350.20 ± 21.84 | mg N-NH3 L−1 |
| Total solids | 17.06 ± 0.56 | 3.05 ± 0.31 | % |
| Total volatile solids | 15.01 ± 0.42 | 2.12 ± 0.12 | % |
| Total fixed solids | 2.05 ± 0.03 | 0.93 ± 0.05 | % |
| Solids biodegradation | – | 82.12 | % |
| Accumulated biogas volume | – | 11,928 | mL |
| Accumulated methane volume | – | 6469.79 | mL |
| Methane yield | – | 10.53 | L CH4 kg−1 TVS |
| Parameters | Modified Gompertz | Cone | First-Order Kinetic |
|---|---|---|---|
| P (mL) | 8427.34 | 7488.87 | 245,819.15 |
| Difference (%) | 30.25 | 15.75 | >100 |
| Rm (mL h−1) | 8.189 | – | – |
| λ (h) | 0.00 | – | – |
| (h−1) | – | 0.0022 | 0.00 |
| n | – | 3.006 | – |
| R2 | 0.945 | 0.992 | 0.949 |
| Adjusted R2 | 0.938 | 0.991 | 0.947 |
| SEE | 621.91 | 234.3 | 580.94 |
| RMSE | 575.78 | 216.92 | 552.58 |
| Parameters | Results | Unit |
|---|---|---|
| 0.133 | MWh ton−1 BSG | |
| 598.45 | MJ ton−1 BSG | |
| 0.0099 | tCO2eq ton−1 BSG | |
| 0.0335 | tCO2eq ton−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sganzerla, W.G.; Costa, J.M.; Tena-Villares, M.; Buller, L.S.; Mussatto, S.I.; Forster-Carneiro, T. Dry Anaerobic Digestion of Brewer’s Spent Grains toward a More Sustainable Brewery: Operational Performance, Kinetic Analysis, and Bioenergy Potential. Fermentation 2023, 9, 2. https://doi.org/10.3390/fermentation9010002
Sganzerla WG, Costa JM, Tena-Villares M, Buller LS, Mussatto SI, Forster-Carneiro T. Dry Anaerobic Digestion of Brewer’s Spent Grains toward a More Sustainable Brewery: Operational Performance, Kinetic Analysis, and Bioenergy Potential. Fermentation. 2023; 9(1):2. https://doi.org/10.3390/fermentation9010002
Chicago/Turabian StyleSganzerla, William Gustavo, Josiel Martins Costa, Miriam Tena-Villares, Luz Selene Buller, Solange I. Mussatto, and Tania Forster-Carneiro. 2023. "Dry Anaerobic Digestion of Brewer’s Spent Grains toward a More Sustainable Brewery: Operational Performance, Kinetic Analysis, and Bioenergy Potential" Fermentation 9, no. 1: 2. https://doi.org/10.3390/fermentation9010002