Comparison of the Chemical Properties of Pineapple Vinegar and Mixed Pineapple and Dragon Fruit Vinegar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Fruit Juices
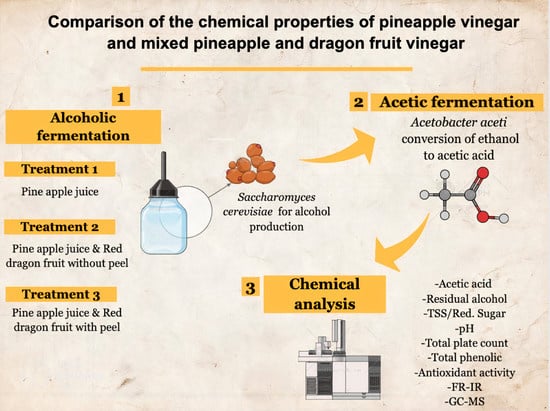
2.2. Alcoholic Fermentation
2.3. Vinegar Fermentation
2.4. Chemical Analysis
2.5. Determination of Total Phenolic Compounds
2.6. Determination of Antioxidant Activity
2.7. Fourier Transform Infrared (FTIR) Analysis
2.8. Gas Chromatography-Mass Spectrometer Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Chemical Characteristics
3.2. Alcoholic Fermentation
3.3. Vinegar Fermentation
3.4. Total Phenolic Content and Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: A meta-analysis. JAMA 2002, 288, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Kitts, D.D. Studies on the antioxidant activity of Echinacea root extract. J. Agri. Food Chem. 2000, 48, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, L.B.; Escobar, J.D.; Sánchez, B.N.; Hughes, R.; Zuniga, B.G.; Garcia, N.; Lisabeth, L.D. Fast food and neighborhood stroke risk. Ann. Neurol. 2009, 66, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Khwanmuang, W. Pretreatment and Production of Red Dragon Fruit Hylocereus polyrhizus Wine. Master’s Thesis, Faculty of Science, Chulalongkorn University, Bangkok, Thailand, 2014. [Google Scholar]
- Thai Ministry of Public Health, (No. 204) B.E. 2543 (2000) Re: Vinegar. Available online: https://food.fda.moph.go.th/law/announ_moph201-250.php (accessed on 10 September 2022).
- All-Around Benefits of Vinegar. Available online: https://www.pobpad.com/%E0%B8%99%E0%B9%89%E0%B8%B3%E0%B8%AA%E0%B9%89%E0%B8%A1%E0%B8%AA%E0%B8%B2%E0%B8%A2%E0%B8%8A%E0%B8%B9-%E0%B9%80%E0%B8%84%E0%B8%A3%E0%B8%B7%E0%B9%88%E0%B8%AD%E0%B8%87%E0%B8%9B%E0%B8%A3%E0%B8%B8%E0%B8%87 (accessed on 10 September 2022).
- Luzón-Quintana, L.M.; Castro, R.; Durán-Guerrero, E. Biotechnological processes in fruit vinegar production. Foods 2021, 10, 945. [Google Scholar] [CrossRef]
- Ousaaid, D.; Mechchate, H.; Laaroussi, H.; Hano, C.; Bakour, M.; El Ghouizi, A.; Conte, R.; Lyoussi, B.; El Arabi, I. Fruits vinegar: Quality characteristics, phytochemistry, and functionality. Molecules 2021, 27, 222. [Google Scholar] [CrossRef]
- Lomthong, T.; Saithong, P. Feasibility of Leum Pua glutinous rice substrate for sugar syrup and vinegar production by raw starch degrading enzyme hydrolysis. Int. Food Res. J. 2019, 26, 1515–1523. [Google Scholar]
- Saithong, P.; Nitipan, S.; Permpool, J. Optimization of vinegar production from nipa (Nypa fruticans Wurmb.) sap using surface culture fermentation process. Appl. Food Biotechnol. 2019, 6, 193–200. [Google Scholar]
- Boondaeng, A.; Kasemsumran, S.; Ngowsuwan, K.; Vaithanomsat, P.; Apiwatanapiwat, W.; Trakunjae, C.; Janchai, P.; Jungtheerapanich, S.; Niyomvong, N. Fermentation condition and quality evaluation of pineapple fruit wine. Fermentation 2021, 8, 11. [Google Scholar] [CrossRef]
- Saithong, P.; On-tom, K.; Muangnoi, M. Application of Surface Culture Fermentation Technique in production of pineapple wine vinegar. Proceeding of the 19th Food Innovation Asia Conference 2017, Bangkok, Thailand, 5–17 June 2017. [Google Scholar]
- IFU. Determination of Titratable Acidity. IFU Analysis No. 3. 2007. Available online: https://www.ifu-fruitjuice.com/ (accessed on 31 August 2022).
- IFU. Determination of Volatile Acids. IFU Analysis No. 5. 2005. Available online: https://www.ifu-fruitjuice.com/ (accessed on 31 August 2022).
- Somogyi, M. Notes on sugar determination. J. Biol. Chem. 1952, 195, 19–23. [Google Scholar] [CrossRef]
- Boondaeng, A.; Vaithanomsat, P.; Apiwatanapiwat, W.; Trakunjae, C.; Kongtud, W. Statistical approach for optimization of ethanol production from fast-growing trees: Acacia mangium and Acacia hybrid. Bioresources 2015, 10, 3154–3168. [Google Scholar] [CrossRef] [Green Version]
- Romsoms, N.; Pornsanthia, J.; Rimlumduan, T.; Vechklang, K. The biological activities of kombucha during fermentation process. Naresaun Phayao J 2020, 14, 75–87. [Google Scholar]
- Xie, R.; Tu, M.; Wu, Y.; Adhikari, S. Improvement in HPLC separation of acetic acid and levulinic acid in the profiling of biomass hydrolysate. Bioresour. Technol. 2011, 102, 4938–4942. [Google Scholar] [CrossRef] [PubMed]
- CIE. Colorimetry; Publication CIE 15.2; John Wiley & Sons: Hoboken, NJ, USA, 1986. [Google Scholar]
- Lim, Y.Y.; Lim, T.T.; Tee, J.J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007, 103, 1003–1008. [Google Scholar] [CrossRef]
- Vidal-Gutiérrez, M.; Robles-Zepeda, R.E.; Vilegas, W.; Gonzalez-Aguilar, G.A.; Torres-Moreno, H.; López-Romero, J.C. Phenolic composition and antioxidant activity of Bursera microphylla A. Gray. Ind. Crops Prod. 2020, 152, 112412. [Google Scholar] [CrossRef]
- Augustine, S.K.; Bhavsar, S.P.; Kapadnis, B.P. A non-polyene antifungal antibiotic from Streptomyces albidoflavus PU 23. J. Biosci. 2005, 30, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, P.; Liao, L.; Qin, Y.; Jiang, L.; Liu, Y. Characteristic fingerprints and volatile flavor compound variations in Liuyang Douchi during fermentation via HS-GC-IMS and HS-SPME-GC- MS. Food Chem. 2021, 361, 130055. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donéche, B.; Lonvaud, A. Handbook of Enology: The Microbiology of Wine and Vinifications; John Wiley & Sons: Chichester, UK, 2006; pp. 79–113. [Google Scholar]
- Gong, X.; Yang, Y.; Ma, L.; Peng, S.; Lin, M. Fermentation and characterization of pitaya wine. IOP Conf. Ser. Earth Environ. Sci. 2017, 100, 012029. [Google Scholar]
- Riansa-ngawong, W.; Tipkanon, S. Development of golden rice wine by rice husk. Khon Kaen Agric. J. 2015, 43, 613–622. (In Thai) [Google Scholar]
- Singh, R.; Singh, S. Design and development of batch type acetifier for wine- vinegar production. Indian J. Microbiol. 2007, 47, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Food and Drug Administration. FDA/ORA Compliance Policy Guides, Sec. 525.825 Vinegar, Definitions: Adulteration with Vinegar Eels (CPG 7109.22). 2007. Available online: https://www.fda.gov/ora/compliance_ref/cpg/cpgfod/cpg525-825.html (accessed on 31 August 2022).
- Tanamool, V.; Chantarangsee, M.; Soemphol, W. Simultaneous vinegar fermentation from a pineapple by-product by co-inoculation of yeast and thermotolerant acetic acid bacteria, and their physicochemical properties. 3 Biotech. 2020, 10, 115. [Google Scholar] [CrossRef]
- Sossou, S.K.; Ameyapoh, Y.; Karou, S.D.; de Souza, C. Study of pineapple peelings processing into vinegar by biotechnology. Pak. J. Biol. Sci. 2009, 12, 859–865. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L. Determination of total phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1.1.1–I1.1.8. [Google Scholar]
- Callejón, R.M.; Torija, M.J.; Mas, A.; Morales, M.L.; Troncoso, A.M. Changes of volatile compounds in wine vinegars during their elaboration in barrels made from diferent woods. Food Chem. 2010, 113, 1252–1259. [Google Scholar] [CrossRef]
- Plioni, I.; Bekatorou, A.; Terpou, A.; Mallouchos, A.; Plessas, S.; Koutinas, A.A.; Katechaki, E. Vinegar production from corinthian currants finishing side-stream: Development and comparison of methods based on immobilized acetic acid Bacteria. Foods 2021, 10, 3133. [Google Scholar] [CrossRef]
- Barkociová, M.; Tóth, J.; Sutor, K.; Drobnicka, N.; Wybraniec, S.; Dudík, B.; Bilková, A.; Czigle, S. Betalains in edible fruits of three Cactaceae Taxa—Epiphyllum, Hylocereus, and Opuntia, their LC-MS/MS and FTIR identification and biological activities evaluation. Plants 2021, 10, 2669. [Google Scholar] [CrossRef]
- Rios-Reina, R.; Elcoroaristizabal, S.; Ocana-Gonzalez, J.A.; Garcia-Gonzalez, D.L.; Amigo, J.M.; Callejon, R.M. Characterization and authentication of Spanish PDO wine vinegars using multidimensional fluorescence and chemometrics. Food Chem. 2017, 230, 108–111. [Google Scholar] [CrossRef] [Green Version]
- Cavdaroglu, C.; Ozen, B. Detection of vinegar adulteration with spirit vinegar and acetic acid using UV–visible and Fourier transform infrared spectroscopy. Food Chem. 2022, 379, 132150. [Google Scholar] [CrossRef]
- Gulcin, I.; Kufrevioglu, O.I.; Oktay, M.; Buyukokuroglu, M.E. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J. Ethnopharmacol. 2004, 90, 205–215. [Google Scholar] [CrossRef]
- Soare, J.R.; Dinis, T.C.; Cunha, A.P.; Almeida, L. Antioxidant activities of some extracts of Thymus zygis. Free Radic. Res. 1997, 26, 469–478. [Google Scholar] [CrossRef]




| Treatment | Ratios of Juices and Water | |||
|---|---|---|---|---|
| Pineapple Juice | Red Dragon Fruit Juice | Red Dragon Fruit Peel Juice | Water | |
| T1 | 4 | 0 | 0 | 2 |
| T2 | 2 | 1 | 0 | 3 |
| T3 | 2 | 0.5 | 0.5 | 3 |
| Chemical Characteristics | Value ± SD | ||
|---|---|---|---|
| Pineapple | Dragon Fruit | Dragon Fruit Peel | |
| Total soluble solid (TSS, °Brix) | 13.07 ± 0.12 | 11.93 ± 0.23 | - |
| Reducing sugar (g/L) | 92.13 ± 1.34 | 48.24 ± 2.42 | - |
| pH | 3.58 ± 0.02 | 4.44 ± 0.01 | - |
| Total titratable acidity (%) | 0.286 ± 0.00 | 0.124 ± 0.00 | - |
| Antioxidant activity (µg TE/g) | 198.29 ± 1.51 | 162.60 ± 8.13 | 123.24 ± 0.82 |
| Total phenolic compound (mg GAE/L) | 407.00 ± 8.60 | 256.50 ± 0.58 | 134.95 ± 7.07 |
| Nitrogen content (w/v%) | 0.08 ± 0.01 | 0.027 ± 0.20 | - |
| Samples | Total Phenolic (µg GAE/mL) | Antioxidant DPPH Assay (µg TE/g) | ||
|---|---|---|---|---|
| Day 0 | Day 20 ns | Day 0 | Day 20 | |
| Pineapple vinegar | 282.74 a ± 4.32 | 245.31 ± 2.88 | 143.79 b ± 0.43 | 189.52 b ± 1.53 |
| Pineapple vinegar mixed with red dragon fruit without peel | 121.11 c ± 3.96 | 234.63 ± 6.48 | 156.88 a ± 3.07 | 187.91 b ± 1.31 |
| Pineapple vinegar mixed with red dragon fruit with peel | 186.41 b ± 12.8 | 259.97 ± 9.09 | 131.22 c ± 3.97 | 210.74 a ± 1.61 |
| Assignment Compounds | Retention Time | % Area | % Similarity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | |
| Ethanol | 1.314 | 1.309 | 1.304 | 15.53 ± 0.76 | 19.75 ± 0.16 | 25.24 ± 0.73 | 98.00 ± 0.00 | 98.00 ± 0.00 | 98.00 ± 0.00 |
| Acetic acid | 1.458 | 1.448 | 1.434 | 30.50 ± 0.10 | 28.18 ± 0.14 | 25.73 ± 0.14 | 96.00 ± 0.00 | 96.00 ± 0.00 | 96.00 ± 0.00 |
| Acetic acid ethyl ester | 1.538 | 1.533 | 1.533 | 46.05 ± 0.76 | 46.42 ± 0.10 | 41.78 ± 0.99 | 96.00 ± 0.00 | 96.00 ± 0.00 | 96.00 ± 0.00 |
| Acetic acid formyl ester | 1.887 | 1.885 | 1.885 | 0.25 ± 0.01 | 0.30 ± 0.01 | 0.24 ± 0.11 | 88.00 ± 0.00 | 88.00 ± 0.00 | 90.67 ± 4.62 |
| Isopentyl alcohol | 2.028 | 2.021 | 2.020 | 5.96 ± 0.14 | 4.54 ± 0.06 | 6.86 ± 0.35 | 96.00 ± 0.00 | 96.00 ± 0.00 | 97.00 ± 1.73 |
| Isobutyl acetate | 2.280 | 2.262 | - | 0.30 ± 0.02 | 0.22 ± 0.01 | - | 97.00 ± 0.00 | 97.00 ± 0.00 | - |
| 2,3-Butanediol | 2.345 | - | - | 0.04 ± 0.08 | 0.13 ± 0.00 | - | 93.00 ± 3.47 | - | - |
| 1-Butanol, 3-methyl-, acetate | 3.536 | 3.527 | 3.524 | 1.35 ± 0.03 | 0.60 ± 0.01 | 0.23 ± 0.02 | 97.00 ± 0.00 | 99.00 ± 0.00 | 99.00 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boondaeng, A.; Kasemsumran, S.; Ngowsuwan, K.; Vaithanomsat, P.; Apiwatanapiwat, W.; Trakunjae, C.; Janchai, P.; Jungtheerapanich, S.; Niyomvong, N. Comparison of the Chemical Properties of Pineapple Vinegar and Mixed Pineapple and Dragon Fruit Vinegar. Fermentation 2022, 8, 597. https://doi.org/10.3390/fermentation8110597
Boondaeng A, Kasemsumran S, Ngowsuwan K, Vaithanomsat P, Apiwatanapiwat W, Trakunjae C, Janchai P, Jungtheerapanich S, Niyomvong N. Comparison of the Chemical Properties of Pineapple Vinegar and Mixed Pineapple and Dragon Fruit Vinegar. Fermentation. 2022; 8(11):597. https://doi.org/10.3390/fermentation8110597
Chicago/Turabian StyleBoondaeng, Antika, Sumaporn Kasemsumran, Kraireuk Ngowsuwan, Pilanee Vaithanomsat, Waraporn Apiwatanapiwat, Chanaporn Trakunjae, Phornphimon Janchai, Sunee Jungtheerapanich, and Nanthavut Niyomvong. 2022. "Comparison of the Chemical Properties of Pineapple Vinegar and Mixed Pineapple and Dragon Fruit Vinegar" Fermentation 8, no. 11: 597. https://doi.org/10.3390/fermentation8110597