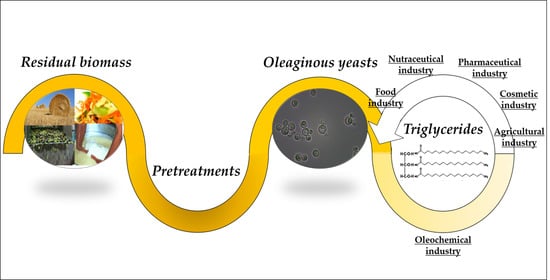
Oleaginous Yeasts as Cell Factories for the Sustainable Production of Microbial Lipids by the Valorization of Agri-Food Wastes
Abstract
:1. Introduction
2. Oleaginous Microorganisms as Cell Factory
3. Oleaginous Microorganisms as Cell Factory
3.1. De Novo Synthesis
3.2. Ex Novo Synthesis
4. Oleaginous Yeasts: Characteristics of Main Species
5. Conversion of Low-Cost Carbon Sources
5.1. Lignocellulosic Agricultural Residues
5.2. Olive Mill Wastewater (OMW)
5.3. Cheese Whey (CW)
5.4. Food Waste (FW)
6. Parameters Affecting Lipogenesis in Oleaginous Yeasts
6.1. Effect of Type and Concentrations of Carbon Source
6.2. Effect of Type and Concentrations of Nitrogen Source
6.3. Effect of Temperature
6.4. Effect of pH
6.5. Effect of Oxygenations
6.6. Effect of Mineral Salts and Other Components
6.7. Factors Affecting the Fatty Acids Profile
7. Applications of Microbial Oils
7.1. Food Applications
7.2. Oleochemical Application
8. Outlook
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vanham, D.; Bouraoui, F.; Leip, A.; Grizzetti, B.; Bidoglio, G. Lost Water and Nitrogen Resources Due to EU Consumer Food Waste. Environ. Res. Lett. 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Diep, N.Q.; Sakanishi, K.; Nakagoshi, N.; Fujimoto, S.; Minowa, T.; Xuan, T.D. Biorefinery: Concepts, Current Status, and Development Trends. Int. J. Ofbiomass Renew. 2012, 1, 1–8. [Google Scholar]
- Probst, K.V.; Schulte, L.R.; Durrett, T.P.; Rezac, M.E.; Vadlani, P.V. Oleaginous Yeast: A Value-Added Platform for Renewable Oils. Crit. Rev. Biotechnol. 2016, 36, 942–955. [Google Scholar]
- Almeida, J.R.M.; Modig, T.; Petersson, A.; Hähn-Hägerdal, B.; Lidén, G.; Gorwa-Grauslund, M.F. Increased Tolerance and Conversion of Inhibitors in Lignocellulosic Hydrolysates by Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. 2007, 82, 340–349. [Google Scholar]
- Ficinus, O. Ueber Den Fettgehalt Des Mutterkorn. Arch. Der Pharm. 1873, 203. [Google Scholar] [CrossRef] [Green Version]
- Nägeli, C.; Loew, O. Ueber Die Chemische Zusammensetzung Der Hefe. Justus Liebigs Ann. Der Chem. 1878, 193, 322–348. [Google Scholar] [CrossRef] [Green Version]
- Bonatsos, N.; Marazioti, C.; Moutousidi, E.; Anagnostou, A.; Koutinas, A.; Kookos, I.K. Techno-Economic Analysis and Life Cycle Assessment of Heterotrophic Yeast-Derived Single Cell Oil Production Process. Fuel 2020, 264. [Google Scholar] [CrossRef]
- Shields-Menard, S.A.; Amirsadeghi, M.; Sukhbaatar, B.; Revellame, E.; Hernandez, R.; Donaldson, J.R.; French, W.T. Lipid Accumulation by Rhodococcus rhodochrous Grown on Glucose. J. Ind. Microbiol. Biotechnol. 2015, 42. [Google Scholar] [CrossRef]
- Röttig, A.; Hauschild, P.; Madkour, M.H.; Al-Ansari, A.M.; Almakishah, N.H.; Steinbüchel, A. Analysis and Optimization of Triacylglycerol Synthesis in Novel Oleaginous Rhodococcus and Streptomyces Strains Isolated from Desert Soil. J. Biotechnol. 2016, 225. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Xia, L. An Oleaginous Endophyte Bacillus subtilis HB1310 Isolated from Thin-Shelled Walnut and Its Utilization of Cotton Stalk Hydrolysate for Lipid Production. Biotechnol. Biofuels 2014, 7. [Google Scholar] [CrossRef] [Green Version]
- Santala, S.; Efimova, E.; Kivinen, V.; Larjo, A.; Aho, T.; Karp, M.; Santala, V. Improved Triacylglycerol Production in Acinetobacter baylyi ADP1 by Metabolic Engineering. Microb. Cell Factories 2011, 10. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Rathour, R.; Gupta, J.; Pandey, A.; Gnansounou, E.; Thakur, I.S. Bacterial production of fatty acid and biodiesel: Opportunity and challenges. In Refining Biomass Residues for Sustainable Energy and Bioproducts: Technology, Advances, Life Cycle Assessment, and Economics; Academic Press: Cambridge, MA, USA, 2020; pp. 21–49. [Google Scholar] [CrossRef]
- Ai, M.; Zhu, Y.; Jia, X. Recent Advances in Constructing Artificial Microbial Consortia for the Production of Medium-Chain-Length Polyhydroxyalkanoates. World J. Microbiol. Biotechnol. 2021, 37, 1–14. [Google Scholar]
- Ferreira, J.A.; Lennartsson, P.R.; Edebo, L.; Taherzadeh, M.J. Zygomycetes-Based Biorefinery: Present Status and Future Prospects. Bioresour. Technol. 2013, 135. [Google Scholar] [CrossRef] [Green Version]
- Hui, L.; Wan, C.; Hai-Tao, D.; Xue-Jiao, C.; Qi-Fa, Z.; Yu-Hua, Z. Direct Microbial Conversion of Wheat Straw into Lipid by a Cellulolytic Fungus of Aspergillus oryzae A-4 in Solid-State Fermentation. Bioresour. Technol. 2010, 101. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Sources of Microbial Oils with Emphasis to Mortierella (Umbelopsis) isabellina Fungus. World J. Microbiol. Biotechnol. 2019, 35, 63. [Google Scholar]
- Ratledge, C.; Wynn, J.P. The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv. Appl. Microbiol. 2002, 51, 1–52. [Google Scholar]
- Troiano, D.; Orsat, V.; Dumont, M.J. Status of Filamentous Fungi in Integrated Biorefineries. Renew. Sustain. Energy Rev. 2020, 117, 109472. [Google Scholar]
- Carota, E.; Crognale, S.; D’Annibale, A.; Petruccioli, M. Bioconversion of Agro-Industrial Waste into Microbial Oils by Filamentous Fungi. Process Saf. Environ. Prot. 2018, 117. [Google Scholar] [CrossRef]
- Ji, X.J.; Huang, H. Engineering Microbes to Produce Polyunsaturated Fatty Acids. Trends Biotechnol. 2019, 37, 344–346. [Google Scholar]
- Liu, Z.; Feist, A.M.; Dragone, G.; Mussatto, S.I. Lipid and Carotenoid Production from Wheat Straw Hydrolysates by Different Oleaginous Yeasts. J. Clean. Prod. 2020, 249. [Google Scholar] [CrossRef]
- Daskalaki, A.; Perdikouli, N.; Aggeli, D.; Aggelis, G. Laboratory Evolution Strategies for Improving Lipid Accumulation in Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2019, 103. [Google Scholar] [CrossRef]
- Caporusso, A.; de Bari, I.; Valerio, V.; Albergo, R.; Liuzzi, F. Conversion of Cardoon Crop Residues into Single Cell Oils by Lipomyces tetrasporus and Cutaneotrichosporon curvatus: Process Optimizations to Overcome the Microbial Inhibition of Lignocellulosic Hydrolysates. Ind. Crop. Prod. 2021, 159. [Google Scholar] [CrossRef]
- Feng, P.; Xu, Z.; Qin, L.; Asraful Alam, M.; Wang, Z.; Zhu, S. Effects of Different Nitrogen Sources and Light Paths of Flat Plate Photobioreactors on the Growth and Lipid Accumulation of Chlorella Sp. GN1 Outdoors. Bioresour. Technol. 2020, 301. [Google Scholar] [CrossRef]
- Nascimento, I.A.; Cabanelas, I.T.D.; dos Santos, J.N.; Nascimento, M.A.; Sousa, L.; Sansone, G. Biodiesel Yields and Fuel Quality as Criteria for Algal-Feedstock Selection: Effects of CO2-Supplementation and Nutrient Levels in Cultures. Algal Res. 2015, 8. [Google Scholar] [CrossRef]
- Li, D.; Zhao, Y.; Ding, W.; Zhao, P.; Xu, J.W.; Li, T.; Ma, H.; Yu, X. A Strategy for Promoting Lipid Production in Green Microalgae Monoraphidium Sp. QLY-1 by Combined Melatonin and Photoinduction. Bioresour. Technol. 2017, 235. [Google Scholar] [CrossRef]
- Arbib, Z.; Ruiz, J.; Álvarez-Díaz, P.; Garrido-Pérez, C.; Perales, J.A. Capability of Different Microalgae Species for Phytoremediation Processes: Wastewater Tertiary Treatment, CO2 Bio-Fixation and Low Cost Biofuels Production. Water Res. 2014, 49. [Google Scholar] [CrossRef]
- Saisriyoot, M.; Thanapimmetha, A.; Suwaleerat, T.; Chisti, Y.; Srinophakun, P. Biomass and Lipid Production by Rhodococcus opacus PD630 in Molasses-Based Media with and without Osmotic-Stress. J. Biotechnol. 2019, 297. [Google Scholar] [CrossRef]
- Salcedo-Vite, K.; Sigala, J.C.; Segura, D.; Gosset, G.; Martinez, A. Acinetobacter baylyi ADP1 Growth Performance and Lipid Accumulation on Different Carbon Sources. Appl. Microbiol. Biotechnol. 2019, 103. [Google Scholar] [CrossRef]
- Gardeli, C.; Athenaki, M.; Xenopoulos, E.; Mallouchos, A.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Lipid Production and Characterization by Mortierella (Umbelopsis) isabellina Cultivated on Lignocellulosic Sugars. J. Appl. Microbiol. 2017, 123. [Google Scholar] [CrossRef]
- Li, S.; Yu, H.; Liu, Y.; Zhang, X.; Ma, F. The Lipid Strategies in Cunninghamella echinulata for an Allostatic Response to Temperature Changes. Process Biochem. 2019, 76. [Google Scholar] [CrossRef]
- Carsanba, E.; Papanikolaou, S.; Fickers, P.; Erten, H. Lipids by Yarrowia lipolytica Strains Cultivated on Glucose in Batch Cultures. Microorganisms 2020, 8, 1054. [Google Scholar] [CrossRef]
- Boviatsi, E.; Papadaki, A.; Efthymiou, M.N.; Nychas, G.J.E.; Papanikolaou, S.; da Silva, J.A.C.; Freire, D.M.G.; Koutinas, A. Valorisation of Sugarcane Molasses for the Production of Microbial Lipids via Fermentation of Two Rhodosporidium Strains for Enzymatic Synthesis of Polyol Esters. J. Chem. Technol. Biotechnol. 2020, 95. [Google Scholar] [CrossRef]
- Di Fidio, N.; Ragaglini, G.; Dragoni, F.; Antonetti, C.; Raspolli Galletti, A.M. Integrated Cascade Biorefinery Processes for the Production of Single Cell Oil by Lipomyces starkeyi from Arundo Donax L. Hydrolysates. Bioresour. Technol. 2021, 325. [Google Scholar] [CrossRef]
- Capusoni, C.; Rodighiero, V.; Cucchetti, D.; Galafassi, S.; Bianchi, D.; Franzosi, G.; Compagno, C. Characterization of Lipid Accumulation and Lipidome Analysis in the Oleaginous Yeasts Rhodosporidium azoricum and Trichosporon oleaginosus. Bioresour. Technol. 2017, 238. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of Oleaginous Yeasts. Part I: Biochemistry of Single Cell Oil Production. Eur. J. Lipid Sci. Technol. 2011, 113, 1031–1051. [Google Scholar]
- Beopoulos, A.; Cescut, J.; Haddouche, R.; Uribelarrea, J.L.; Molina-Jouve, C.; Nicaud, J.M. Yarrowia lipolytica as a Model for Bio-Oil Production. Prog. Lipid Res. 2009, 48, 375–387. [Google Scholar]
- Wu, S.; Hu, C.; Jin, G.; Zhao, X.; Zhao, Z.K. Phosphate-Limitation Mediated Lipid Production by Rhodosporidium toruloides. Bioresour. Technol. 2010, 101. [Google Scholar] [CrossRef]
- Wynn, J.P.; Ratledge, C. Oils from Microorganisms. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Wasylenko, T.M.; Ahn, W.S.; Stephanopoulos, G. The Oxidative Pentose Phosphate Pathway Is the Primary Source of NADPH for Lipid Overproduction from Glucose in Yarrowia lipolytica. Metab. Eng. 2015, 30. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Skerker, J.M.; Rutter, C.D.; Maurer, M.J.; Arkin, A.P.; Rao, C.V. Engineering Rhodosporidium toruloides for Increased Lipid Production. Biotechnol. Bioeng. 2016, 113. [Google Scholar] [CrossRef]
- Arous, F.; Azabou, S.; Triantaphyllidou, I.E.; Aggelis, G.; Jaouani, A.; Nasri, M.; Mechichi, T. Newly Isolated Yeasts from Tunisian Microhabitats: Lipid Accumulation and Fatty Acid Composition. Eng. Life Sci. 2017, 17. [Google Scholar] [CrossRef]
- Qin, L.; Liu, L.; Zeng, A.P.; Wei, D. From Low-Cost Substrates to Single Cell Oils Synthesized by Oleaginous Yeasts. Bioresour. Technol. 2017, 245, 1507–1519. [Google Scholar]
- Jin, M.; Slininger, P.J.; Dien, B.S.; Waghmode, S.; Moser, B.R.; Orjuela, A.; Sousa, L.d.C.; Balan, V. Microbial Lipid-Based Lignocellulosic Biorefinery: Feasibility and Challenges. Trends Biotechnol. 2015, 33, 43–54. [Google Scholar]
- Satyanarayana, T.; Gotthard, K. Yeast Biotechnology: Diversity and Applications, 1st ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Thevenieau, F.; Nicaud, J.M. Microorganisms as Sources of Oils. OCL - Oilseeds Fats Crops Lipids 2013, 20, D603. [Google Scholar]
- Madzak, C. Yarrowia lipolytica: Recent Achievements in Heterologous Protein Expression and Pathway Engineering. Appl. Microbiol. Biotechnol. 2015, 99, 4559–4577. [Google Scholar]
- Liu, N.; Qiao, K.; Stephanopoulos, G. 13C Metabolic Flux Analysis of Acetate Conversion to Lipids by Yarrowia lipolytica. Metab. Eng. 2016, 38. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.; Li, Z.; Zhou, X.; Cheng, S.; Zheng, L. Oleaginous Yeast Yarrowia lipolytica Culture with Synthetic and Food Waste-Derived Volatile Fatty Acids for Lipid Production. Biotechnol. Biofuels 2017, 10. [Google Scholar] [CrossRef]
- Fontanille, P.; Kumar, V.; Christophe, G.; Nouaille, R.; Larroche, C. Bioconversion of Volatile Fatty Acids into Lipids by the Oleaginous Yeast Yarrowia lipolytica. Bioresour. Technol. 2012, 114. [Google Scholar] [CrossRef]
- Silverman, A.M.; Qiao, K.; Xu, P.; Stephanopoulos, G. Functional Overexpression and Characterization of Lipogenesis-Related Genes in the Oleaginous Yeast Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2016, 100. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, L.V.; Coelho, M.A.Z.; Amaral, P.F.F.; Fickers, P. A Novel Osmotic Pressure Strategy to Improve Erythritol Production by Yarrowia lipolytica from Glycerol. Bioprocess Biosyst. Eng. 2018, 41. [Google Scholar] [CrossRef]
- Epova, E.; Guseva, M.; Kovalyov, L.; Isakova, E.; Deryabina, Y.; Belyakova, A.; Zylkova, M.; Shevelev, A. Identification of Proteins Involved in pH Adaptation in Extremophile Yeast Yarrowia lipolytica. In Proteomic applications in biology; Heazlewood, J.L., Petzold, C.J., Eds.; InTech: Rijeka, Croatia, 2012; pp. 209–224. [Google Scholar]
- Walker, C.; Ryu, S.; Trinh, C.T. Exceptional Solvent Tolerance in Yarrowia lipolytica Is Enhanced by Sterols. Metab. Eng. 2019, 54. [Google Scholar] [CrossRef]
- Dujon, B.; Sherman, D.; Fischer, G.; Durrens, P.; Casaregela, S.; Lafentaine, I.; de Montigny, J.; Marck, C.; Neuvéglise, C.; Talla, E.; et al. Genome Evolution in Yeasts. Nature 2004, 430. [Google Scholar] [CrossRef]
- Markham, K.A.; Alper, H.S. Synthetic Biology Expands the Industrial Potential of Yarrowia lipolytica. Trends Biotechnol. 2018, 36, 1085–1095. [Google Scholar]
- Blazeck, J.; Hill, A.; Liu, L.; Knight, R.; Miller, J.; Pan, A.; Otoupal, P.; Alper, H.S. Harnessing Yarrowia lipolytica Lipogenesis to Create a Platform for Lipid and Biofuel Production. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Xie, D.; Jackson, E.N.; Zhu, Q. Sustainable Source of Omega-3 Eicosapentaenoic Acid from Metabolically Engineered Yarrowia lipolytica: From Fundamental Research to Commercial Production. Appl. Microbiol. Biotechnol. 2015, 99, 1599–1610. [Google Scholar]
- Papanikolaou, S.; Aggelis, G. Lipid Production by Yarrowia lipolytica Growing on Industrial Glycerol in a Single-Stage Continuous Culture. Bioresour. Technol. 2002, 82. [Google Scholar] [CrossRef]
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; van Dijck, P.W.M.; Wyss, M. Yarrowia lipolytica: Safety Assessment of an Oleaginous Yeast with a Great Industrial Potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar]
- Ferreira, P.; Lopes, M.; Mota, M.; Belo, I. Oxygen Mass Transfer Impact on Citric Acid Production by Yarrowia lipolytica from Crude Glycerol. Biochem. Eng. J. 2016, 110. [Google Scholar] [CrossRef] [Green Version]
- Rywińska, A.; Musiał, I.; Rymowicz, W.; Arowska, B.; Boruczkowski, T. Effect of Agitation and Aeration on the Citric Acid Production by Yarrowia lipolytica Grown on Glycerol. Prep. Biochem. Biotechnol. 2012, 42. [Google Scholar] [CrossRef]
- Rzechonek, D.A.; Dobrowolski, A.; Rymowicz, W.; Mirończuk, A.M. Aseptic Production of Citric and Isocitric Acid from Crude Glycerol by Genetically Modified Yarrowia lipolytica. Bioresour. Technol. 2019, 271. [Google Scholar] [CrossRef]
- Rakicka, M.; Rywińska, A.; Cybulski, K.; Rymowicz, W. Enhanced Production of Erythritol and Mannitol by Yarrowia lipolytica in Media Containing Surfactants. Braz. J. Microbiol. 2016, 47. [Google Scholar] [CrossRef] [Green Version]
- Dulermo, T.; Lazar, Z.; Dulermo, R.; Rakicka, M.; Haddouche, R.; Nicaud, J.M. Analysis of ATP-Citrate Lyase and Malic Enzyme Mutants of Yarrowia lipolytica Points out the Importance of Mannitol Metabolism in Fatty Acid Synthesis. Biochim. Et Biophys. Acta -Mol. Cell Biol. Lipids 2015, 1851. [Google Scholar] [CrossRef] [Green Version]
- Carsanba, E.; Papanikolaou, S.; Fickers, P.; Erten, H. Screening Various Yarrowia lipolytica Strains for Citric Acid Production. Yeast 2019, 36. [Google Scholar] [CrossRef]
- LI, Y.H.; LIU, B.; ZHAO, Z.B.; BAI, F.W. Optimization of Culture Conditions for Lipid Production by Rhodosporidium toruloides. Chin. J. Biotechnol. 2006, 22. [Google Scholar] [CrossRef]
- Yaegashi, J.; Kirby, J.; Ito, M.; Sun, J.; Dutta, T.; Mirsiaghi, M.; Sundstrom, E.R.; Rodriguez, A.; Baidoo, E.; Tanjore, D.; et al. Rhodosporidium toruloides: A New Platform Organism for Conversion of Lignocellulose into Terpene Biofuels and Bioproducts. Biotechnol. Biofuels 2017, 10. [Google Scholar] [CrossRef] [Green Version]
- Tsakona, S.; Papadaki, A.; Kopsahelis, N.; Kachrimanidou, V.; Papanikolaou, S.; Koutinas, A. Development of a Circular Oriented Bioprocess for Microbial Oil Production Using Diversified Mixed Confectionery Side-Streams. Foods 2019, 8, 300. [Google Scholar] [CrossRef] [Green Version]
- Tchakouteu, S.S.; Kalantzi, O.; Gardeli, C.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Lipid Production by Yeasts Growing on Biodiesel-Derived Crude Glycerol: Strain Selection and Impact of Substrate Concentration on the Fermentation Efficiency. J. Appl. Microbiol. 2015, 118. [Google Scholar] [CrossRef]
- Huang, X.F.; Liu, J.N.; Lu, L.J.; Peng, K.M.; Yang, G.X.; Liu, J. Culture Strategies for Lipid Production Using Acetic Acid as Sole Carbon Source by Rhodosporidium toruloides. Bioresour. Technol. 2016, 206. [Google Scholar] [CrossRef]
- Sundstrom, E.; Yaegashi, J.; Yan, J.; Masson, F.; Papa, G.; Rodriguez, A.; Mirsiaghi, M.; Liang, L.; He, Q.; Tanjore, D.; et al. Demonstrating a Separation-Free Process Coupling Ionic Liquid Pretreatment, Saccharification, and Fermentation with: Rhodosporidium toruloides to Produce Advanced Biofuels. Green Chem. 2018, 20. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Liu, D. Exploitation of Genus Rhodosporidium for Microbial Lipid Production. World J. Microbiol. Biotechnol. 2017, 33, 54. [Google Scholar]
- SànchezNogué, V.; Black, B.A.; Kruger, J.S.; Singer, C.A.; Ramirez, K.J.; Reed, M.L.; Cleveland, N.S.; Singer, E.R.; Yi, X.; Yeap, R.Y.; et al. Integrated Diesel Production from Lignocellulosic Sugars: Via Oleaginous Yeast. Green Chem. 2018, 20, 4349–4365. [Google Scholar] [CrossRef]
- Singh, G.; Sinha, S.; Bandyopadhyay, K.K.; Lawrence, M.; Paul, D. Triauxic Growth of an Oleaginous Red Yeast Rhodosporidium toruloides on Waste “extract” for Enhanced and Concomitant Lipid and β-Carotene Production. Microb. Cell Factories 2018, 17. [Google Scholar] [CrossRef]
- Lopes, H.J.S.; Bonturi, N.; Miranda, E.A. Rhodotorula toruloides Single Cell Oil Production Using Eucalyptus Urograndis Hemicellulose Hydrolysate as a Carbon Source. Energies 2020, 13, 795. [Google Scholar] [CrossRef] [Green Version]
- Wen, Z.; Zhang, S.; Odoh, C.K.; Jin, M.; Zhao, Z.K. Rhodosporidium toruloides—A Potential Red Yeast Chassis for Lipids and Beyond. FEMS Yeast Res. 2020, 20, foaa038. [Google Scholar]
- Zhu, Z.; Zhang, S.; Liu, H.; Shen, H.; Lin, X.; Yang, F.; Zhou, Y.J.; Jin, G.; Ye, M.; Zou, H.; et al. A Multi-Omic Map of the Lipid-Producing Yeast Rhodosporidium toruloides. Nat. Commun. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Sutanto, S.; Zullaikah, S.; Tran-Nguyen, P.L.; Ismadji, S.; Ju, Y.H. Lipomyces starkeyi: Its Current Status as a Potential Oil Producer. Fuel Process. Technol. 2018, 177, 39–55. [Google Scholar]
- Angerbauer, C.; Siebenhofer, M.; Mittelbach, M.; Guebitz, G.M. Conversion of Sewage Sludge into Lipids by Lipomyces starkeyi for Biodiesel Production. Bioresour. Technol. 2008, 99. [Google Scholar] [CrossRef]
- Juanssilfero, A.B.; Kahar, P.; Amza, R.L.; Miyamoto, N.; Otsuka, H.; Matsumoto, H.; Kihira, C.; Thontowi, A.; Ogino, C.; Prasetya, B.; et al. Effect of Inoculum Size on Single-Cell Oil Production from Glucose and Xylose Using Oleaginous Yeast Lipomyces starkeyi. J. Biosci. Bioeng. 2018, 125, 695–702. [Google Scholar] [CrossRef]
- Di Fidio, N.; Dragoni, F.; Antonetti, C.; de Bari, I.; Raspolli Galletti, A.M.; Ragaglini, G. From Paper Mill Waste to Single Cell Oil: Enzymatic Hydrolysis to Sugars and Their Fermentation into Microbial Oil by the Yeast Lipomyces starkeyi. Bioresour. Technol. 2020, 315. [Google Scholar] [CrossRef]
- Liu, L.p.; Zong, M.h.; Hu, Y.; Li, N.; Lou, W.y.; Wu, H. Efficient Microbial Oil Production on Crude Glycerol by Lipomyces starkeyi AS 2.1560 and Its Kinetics. Process Biochem. 2017, 58. [Google Scholar] [CrossRef]
- Gou, Q.; Tang, M.; Wang, Y.; Zhou, W.; Liu, Y.; Gong, Z. Deficiency of β-Glucosidase Beneficial for the Simultaneous Saccharification and Lipid Production by the Oleaginous Yeast Lipomyces starkeyi. Appl. Biochem. Biotechnol. 2020, 190. [Google Scholar] [CrossRef]
- Xavier, M.C.A.; Coradini, A.L.V.; Deckmann, A.C.; Franco, T.T. Lipid Production from Hemicellulose Hydrolysate and Acetic Acid by Lipomyces starkeyi and the Ability of Yeast to Metabolize Inhibitors. Biochem. Eng. J. 2017, 118. [Google Scholar] [CrossRef]
- Riley, R.; Haridas, S.; Wolfe, K.H.; Lopes, M.R.; Hittinger, C.T.; Göker, M.; Salamov, A.A.; Wisecaver, J.H.; Long, T.M.; Calvey, C.H.; et al. Comparative Genomics of Biotechnologically Important Yeasts. Proc. Natl. Acad. Sci. USA 2016, 113. [Google Scholar] [CrossRef] [Green Version]
- Takaku, H.; Ebina, S.; Kasuga, K.; Sato, R.; Ara, S.; Kazama, H.; Matsuzawa, T.; Yaoi, K.; Araki, H.; Shida, Y.; et al. Isolation and Characterization of Lipomyces starkeyi Mutants with Greatly Increased Lipid Productivity Following UV Irradiation. J. Biosci. Bioeng. 2021. [Google Scholar] [CrossRef]
- Matsuzawa, T.; Maehara, T.; Kamisaka, Y.; Ara, S.; Takaku, H.; Yaoi, K. Identification and Characterization of Δ12 and Δ12/Δ15 Bifunctional Fatty Acid Desaturases in the Oleaginous Yeast Lipomyces starkeyi. Appl. Microbiol. Biotechnol. 2018, 102. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, X.; Zhu, X.L.; Li, Y.; Xu, H.P.; Zhao, B.F.; Chen, L.; Zhang, X.D. Microbial Lipid Production by the Oleaginous Yeast Cryptococcus curvatus O3 Grown in Fed-Batch Culture. Biomass Bioenergy 2011, 35. [Google Scholar] [CrossRef]
- Di Fidio, N.; Liuzzi, F.; Mastrolitti, S.; Albergo, R.; de Bari, I. Single Cell Oil Production from Undetoxified Arundo Donax L. Hydrolysate by Cutaneotrichosporon curvatus. J. Microbiol. Biotechnol. 2019, 29. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Yuan, M.; Liu, J.N.; Huang, X.F. Bioconversion of Mixed Volatile Fatty Acids into Microbial Lipids by Cryptococcus curvatus ATCC 20509. Bioresour. Technol. 2017, 241. [Google Scholar] [CrossRef]
- Liu, J.; Mu, T.; He, W.; He, T.; Lu, L.; Peng, K.; Huang, X. Integration of Coagulation, Acid Separation and Struvite Precipitation as Fermentation Medium Conditioning Methods to Enhance Microbial Lipid Production from Dewatered Sludge. Bioresour. Technol. Rep. 2019, 7. [Google Scholar] [CrossRef]
- Meng, X.; Yang, J.; Xu, X.; Zhang, L.; Nie, Q.; Xian, M. Biodiesel Production from Oleaginous Microorganisms. Renew. Energy 2009, 34, 1–5. [Google Scholar]
- Hofmeyer, T.; Hackenschmidt, S.; Nadler, F.; Thürmer, A.; Daniel, R.; Kabisch, J. Draft Genome Sequence of Cutaneotrichosporon curvatus DSM 101032 (Formerly Cryptococcus curvatus), an Oleaginous Yeast Producing Polyunsaturated Fatty Acids. Genome Announc. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Wagner, J.M.; Alper, H.S. Synthetic Biology and Molecular Genetics in Non-Conventional Yeasts: Current Tools and Future Advances. Fungal Genet. Biol. 2016, 89. [Google Scholar] [CrossRef] [Green Version]
- Gujjari, P.; Suh, S.O.; Coumes, K.; Zhou, J.J. Characterization of Oleaginous Yeasts Revealed Two Novel Species: Trichosporon cacaoliposimilis Sp. Nov. and Trichosporon oleaginosus Sp. Nov. Mycologia 2011, 103. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Idossou, V.; Tyagi, R.D.; Li, J.; Wang, H. Lipid Accumulation from Trichosporon oleaginosus with Co-Fermentation of Washed Wastewater Sludge and Crude Glycerol. Fuel 2018, 226. [Google Scholar] [CrossRef]
- Šantek, M.I.; Lisičar, J.; Mušak, L.; Špoljarić, I.V.; Beluhan, S.; Šantek, B. Lipid Production by Yeast Trichosporon oleaginosus on the Enzymatic Hydrolysate of Alkaline Pretreated Corn Cobs for Biodiesel Production. Energy Fuels 2018, 32. [Google Scholar] [CrossRef]
- Yu, X.; Zheng, Y.; Dorgan, K.M.; Chen, S. Oil Production by Oleaginous Yeasts Using the Hydrolysate from Pretreatment of Wheat Straw with Dilute Sulfuric Acid. Bioresour. Technol. 2011, 102. [Google Scholar] [CrossRef]
- Kourist, R.; Bracharz, F.; Lorenzen, J.; Kracht, O.N.; Chovatia, M.; Daum, C.; Deshpande, S.; Lipzen, A.; Nolan, M.; Ohm, R.A.; et al. Genomics and Transcriptomics Analyses of the Oil-Accumulating Basidiomycete Yeast Trichosporon oleaginosus: Insights into Substrate Utilization and Alternative Evolutionary Trajectories of Fungal Mating Systems. mBio 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Ageitos, J.M.; Vallejo, J.A.; Veiga-Crespo, P.; Villa, T.G. Oily Yeasts as Oleaginous Cell Factories. Appl. Microbiol. Biotechnol. 2011, 90, 1219–1227. [Google Scholar]
- Chen, H.Z.; Liu, Z.H. Enzymatic Hydrolysis of Lignocellulosic Biomass from Low to High Solids Loading. Eng. Life Sci. 2017, 17, 489–499. [Google Scholar]
- Kumar, A.K.; Sharma, S. Recent Updates on Different Methods of Pretreatment of Lignocellulosic Feedstocks: A Review. Bioresour. Bioprocess. 2017, 4, 1–19. [Google Scholar]
- Capolupo, L.; Faraco, V. Green Methods of Lignocellulose Pretreatment for Biorefinery Development. Appl. Microbiol. Biotechnol. 2016, 100, 9451–9467. [Google Scholar]
- Singh, R.; Krishna, B.B.; Kumar, J.; Bhaskar, T. Opportunities for Utilization of Non-Conventional Energy Sources for Biomass Pretreatment. Bioresour. Technol. 2016, 199, 398–407. [Google Scholar]
- De Bari, I.; Liuzzi, F.; Villone, A.; Braccio, G. Hydrolysis of Concentrated Suspensions of Steam Pretreated Arundo Donax. Appl. Energy 2013, 102. [Google Scholar] [CrossRef]
- Ravindran, R.; Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. A Review on Bioconversion of Agro-Industrial Wastes to Industrially Important Enzymes. Bioengineering 2018, 5, 93. [Google Scholar]
- Kim, D. Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review. Molecules 2018, 23, 309. [Google Scholar]
- Nichols, N.N.; Sharma, L.N.; Mowery, R.A.; Chambliss, C.K.; van Walsum, G.P.; Dien, B.S.; Iten, L.B. Fungal Metabolism of Fermentation Inhibitors Present in Corn Stover Dilute Acid Hydrolysate. Enzym. Microb. Technol. 2008, 42. [Google Scholar] [CrossRef]
- Cao, G.; Ximenes, E.; Nichols, N.N.; Frazer, S.E.; Kim, D.; Cotta, M.A.; Ladisch, M. Bioabatement with Hemicellulase Supplementation to Reduce Enzymatic Hydrolysis Inhibitors. Bioresour. Technol. 2015, 190. [Google Scholar] [CrossRef]
- Tsigie, Y.A.; Wang, C.Y.; Truong, C.T.; Ju, Y.H. Lipid Production from Yarrowia lipolytica Po1g Grown in Sugarcane Bagasse Hydrolysate. Bioresour. Technol. 2011, 102. [Google Scholar] [CrossRef]
- Kim, Y.; Ximenes, E.; Mosier, N.S.; Ladisch, M.R. Soluble Inhibitors/Deactivators of Cellulase Enzymes from Lignocellulosic Biomass. Enzym. Microb. Technol. 2011, 48. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Ladisch, M.R.; Engelberth, A.S. Acetic Acid Removal from Corn Stover Hydrolysate Using Ethyl Acetate and the Impact on Saccharomyces cerevisiae Bioethanol Fermentation. Biotechnol. Prog. 2016, 32. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.O. Bioconversion of Lignocellulose: Inhibitors and Detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar]
- Hicks, R.H.; Sze, Y.; Chuck, C.J.; Henk, D.A. Enhanced Inhibitor Tolerance and Increased Lipid Productivity through Adaptive Laboratory Evolution in the Oleaginous Yeast Metshnikowia pulcherrima. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Sitepu, I.; Selby, T.; Lin, T.; Zhu, S.; Boundy-Mills, K. Carbon Source Utilization and Inhibitor Tolerance of 45 Oleaginous Yeast Species. J. Ind. Microbiol. Biotechnol. 2014, 41. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Zeng, J.; Zheng, Y.; Chen, S. Effect of Lignocellulose Degradation Products on Microbial Biomass and Lipid Production by the Oleaginous Yeast Cryptococcus curvatus. Process Biochem. 2014, 49. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, X.; Zhao, J.; Wu, S.; Zhao, Z.K. Effects of Biomass Hydrolysis By-Products on Oleaginous Yeast Rhodosporidium toruloides. Bioresour. Technol. 2009, 100. [Google Scholar] [CrossRef]
- Poontawee, R.; Limtong, S. Feeding Strategies of Two-Stage Fed-Batch Cultivation Processes for Microbial Lipid Production from Sugarcane Top Hydrolysate and Crude Glycerol by the Oleaginous Red Yeast Rhodosporidiobolus fluvialis. Microorganisms 2020, 8, 151. [Google Scholar] [CrossRef] [Green Version]
- Favaro, L.; Corte, L.; Roscini, L.; Cagnin, L.; Tiecco, M.; Colabella, C.; Berti, A.; Basaglia, M.; Cardinali, G.; Casella, S. A Novel FTIR-Based Approach to Evaluate the Interactions between Lignocellulosic Inhibitory Compounds and Their Effect on Yeast Metabolism. Rsc Adv. 2016, 6. [Google Scholar] [CrossRef]
- Huang, X.f.; Shen, Y.; Luo, H.j.; Liu, J.n.; Liu, J. Enhancement of Extracellular Lipid Production by Oleaginous Yeast through Preculture and Sequencing Batch Culture Strategy with Acetic Acid. Bioresour. Technol. 2018, 247. [Google Scholar] [CrossRef]
- Miranda, C.; Bettencourt, S.; Pozdniakova, T.; Pereira, J.; Sampaio, P.; Franco-Duarte, R.; Pais, C. Modified High-Throughput Nile Red Fluorescence Assay for the Rapid Screening of Oleaginous Yeasts Using Acetic Acid as Carbon Source. Bmc Microbiol. 2020, 20. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Q.; Zhang, H.; Bao, J. Inhibitor Degradation and Lipid Accumulation Potentials of Oleaginous Yeast Trichosporon cutaneum Using Lignocellulose Feedstock. Bioresour. Technol. 2016, 218. [Google Scholar] [CrossRef]
- Díaz, T.; Fillet, S.; Campoy, S.; Vázquez, R.; Viña, J.; Murillo, J.; Adrio, J.L. Combining Evolutionary and Metabolic Engineering in Rhodosporidium toruloides for Lipid Production with Non-Detoxified Wheat Straw Hydrolysates. Appl. Microbiol. Biotechnol. 2018, 102. [Google Scholar] [CrossRef]
- Ananthi, V.; Siva Prakash, G.; Chang, S.W.; Ravindran, B.; Nguyen, D.D.; Vo, D.V.N.; La, D.D.; Bach, Q.V.; Wong, J.W.C.; Kumar Gupta, S.; et al. Enhanced Microbial Biodiesel Production from Lignocellulosic Hydrolysates Using Yeast Isolates. Fuel 2019, 256. [Google Scholar] [CrossRef]
- Lee, J.-E.; Vadlani, P.v.; Min, D. Sustainable Production of Microbial Lipids from Lignocellulosic Biomass Using Oleaginous Yeast Cultures. J. Sustain. Bioenergy Syst. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Diwan, B.; Parkhey, P.; Gupta, P. From Agro-Industrial Wastes to Single Cell Oils: A Step towards Prospective Biorefinery. Folia Microbiol. 2018, 63, 547–568. [Google Scholar]
- Dien, B.S.; Zhu, J.Y.; Slininger, P.J.; Kurtzman, C.P.; Moser, B.R.; O’Bryan, P.J.; Gleisner, R.; Cotta, M.A. Conversion of SPORL Pretreated Douglas Fir Forest Residues into Microbial Lipids with Oleaginous Yeasts. RSC Adv. 2016, 6, 20695–20705. [Google Scholar] [CrossRef]
- Miao, Z.; Tian, X.; Liang, W.; He, Y.; Wang, G. Bioconversion of Corncob Hydrolysate into Microbial Lipid by an Oleaginous Yeast Rhodotorula taiwanensis AM2352 for Biodiesel Production. Renew. Energy 2020, 161, 91–97. [Google Scholar] [CrossRef]
- Fei, Q.; O’Brien, M.; Nelson, R.; Chen, X.; Lowell, A.; Dowe, N. Enhanced Lipid Production by Rhodosporidium toruloides Using Different Fed-Batch Feeding Strategies with Lignocellulosic Hydrolysate as the Sole Carbon Source. Biotechnol. Biofuels 2016, 9. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Xu, Z.; Chen, S.; Jin, M. Microbial Lipid Production from Dilute Acid and Dilute Alkali Pretreated Corn Stover via Trichosporon dermatis. Bioresour. Technol. 2020, 295. [Google Scholar] [CrossRef]
- Antonopoulou, I.; Spanopoulos, A.; Matsakas, L. Single Cell Oil and Ethanol Production by the Oleaginous Yeast Trichosporon fermentans Utilizing Dried Sweet Sorghum Stalks. Renew. Energy 2020, 146, 1609–1617. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive Mill Wastes: Biochemical Characterizations and Valorization Strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar]
- Lee, Z.S.; Chin, S.Y.; Lim, J.W.; Witoon, T.; Cheng, C.K. Treatment Technologies of Palm Oil Mill Effluent (POME) and Olive Mill Wastewater (OMW): A Brief Review. Environ. Technol. Innov. 2019, 15, 100377. [Google Scholar]
- Batuecas, E.; Tommasi, T.; Battista, F.; Negro, V.; Sonetti, G.; Viotti, P.; Fino, D.; Mancini, G. Life Cycle Assessment of Waste Disposal from Olive Oil Production: Anaerobic Digestion and Conventional Disposal on Soil. J. Environ. Manag. 2019, 237. [Google Scholar] [CrossRef]
- Magdich, S.; Abid, W.; Boukhris, M.; ben Rouina, B.; Ammar, E. Effects of Long-Term Olive Mill Wastewater Spreading on the Physiological and Biochemical Responses of Adult Chemlali Olive Trees (Olea Europaea L.). Ecol. Eng. 2016, 97. [Google Scholar] [CrossRef]
- Karaouzas, I.; Skoulikidis, N.T.; Giannakou, U.; Albanis, T.A. Spatial and Temporal Effects of Olive Mill Wastewaters to Stream Macroinvertebrates and Aquatic Ecosystems Status. Water Res. 2011, 45. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Pimentel-Moral, S.; Verardo, V.; Martinez-Ferez, A. A Focus on Advanced Physico-Chemical Processes for Olive Mill Wastewater Treatment. Sep. Purif. Technol. 2017, 179, 161–174. [Google Scholar]
- Orive, M.; Cebrián, M.; Zufía, J. Techno-Economic Anaerobic Co-Digestion Feasibility Study for Two-Phase Olive Oil Mill Pomace and Pig Slurry. Renew. Energy 2016, 97. [Google Scholar] [CrossRef]
- Muktadirul Bari Chowdhury, A.K.M.; Akratos, C.S.; Vayenas, D.v.; Pavlou, S. Olive Mill Waste Composting: A Review. Int. Biodeterior. Biodegrad. 2013, 85, 108–119. [Google Scholar]
- MacCi, C.; Masciandaro, G.; Ceccanti, B. Vermicomposting of Olive Oil Mill Wastewaters. Waste Manag. Res. 2010, 28. [Google Scholar] [CrossRef]
- Tortosa, G.; Alburquerque, J.A.; Ait-Baddi, G.; Cegarra, J. The Production of Commercial Organic Amendments and Fertilisers by Composting of Two-Phase Olive Mill Waste (“alperujo”). J. Clean. Prod. 2012, 26. [Google Scholar] [CrossRef]
- Lopes, M.; Araújo, C.; Aguedo, M.; Gomes, N.; Gonçalves, C.; Teixeira, J.A.; Belo, I. The Use of Olive Mill Wastewater by Wild Type Yarrowia lipolytica Strains: Medium Supplementation and Surfactant Presence Effect. J. Chem. Technol. Biotechnol. 2009, 84. [Google Scholar] [CrossRef] [Green Version]
- Lanciotti, R.; Gianotti, A.; Baldi, D.; Angrisani, R.; Suzzi, G.; Mastrocola, D.; Guerzoni, M.E. Use of Yarrowia lipolytica Strains for the Treatment of Olive Mill Wastewater. Bioresour. Technol. 2005, 96. [Google Scholar] [CrossRef]
- Sarris, D.; Stoforos, N.G.; Mallouchos, A.; Kookos, I.K.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Production of Added-Value Metabolites by Yarrowia lipolytica Growing in Olive Mill Wastewater-Based Media under Aseptic and Non-Aseptic Conditions. Eng. Life Sci. 2017, 17. [Google Scholar] [CrossRef] [Green Version]
- Sarris, D.; Galiotou-Panayotou, M.; Koutinas, A.A.; Komaitis, M.; Papanikolaou, S. Citric Acid, Biomass and Cellular Lipid Production by Yarrowia lipolytica Strains Cultivated on Olive Mill Wastewater-Based Media. J. Chem. Technol. Biotechnol. 2011, 86, 1439–1448. [Google Scholar] [CrossRef]
- Dourou, M.; Kancelista, A.; Juszczyk, P.; Sarris, D.; Bellou, S.; Triantaphyllidou, I.E.; Rywinska, A.; Papanikolaou, S.; Aggelis, G. Bioconversion of Olive Mill Wastewater into High-Added Value Products. J. Clean. Prod. 2016, 139, 957–969. [Google Scholar] [CrossRef]
- Dias, B.; Lopes, M.; Ramôa, R.; Pereira, A.S.; Belo, I. Candida tropicalis as a Promising Oleaginous Yeast for Olive Mill Wastewater Bioconversion. Energies 2021, 14, 640. [Google Scholar] [CrossRef]
- Xenopoulos, E.; Giannikakis, I.; Chatzifragkou, A.; Koutinas, A.; Papanikolaou, S. Lipid Production by Yeasts Growing on Commercial Xylose in Submerged Cultures with Process Water Being Partially Replaced by Olive Mill Wastewaters. Processes 2020, 8, 819. [Google Scholar] [CrossRef]
- Yousuf, A.; Sannino, F.; Addorisio, V.; Pirozzi, D. Microbial Conversion of Olive Oil Mill Wastewaters into Lipids Suitable for Biodiesel Production. J. Agric. Food Chem. 2010, 58, 8630–8635. [Google Scholar] [CrossRef]
- Jarboui, R.; Magdich, S.; Ayadi, R.J.; Gargouri, A.; Gharsallah, N.; Ammar, E. Aspergillus niger P6 and Rhodotorula mucilaginosa CH4 Used for Olive Mill Wastewater (OMW) Biological Treatment in Single Pure and Successive Cultures. Environ. Technol. 2013, 34. [Google Scholar] [CrossRef]
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese Whey Wastewater: Characterization and Treatment. Sci. Total Environ. 2013, 445–446, 385–396. [Google Scholar]
- Mollea, C.; Marmo, L.; Bosco, F. Valorisation of Cheese Whey, a By-Product from the Dairy Industry. Food Industry 2013. [Google Scholar] [CrossRef] [Green Version]
- Seo, Y.H.; Lee, I.; Jeon, S.H.; Han, J.I. Efficient Conversion from Cheese Whey to Lipid Using Cryptococcus curvatus. Biochem. Eng. J. 2014, 90. [Google Scholar] [CrossRef]
- Taskin, M.; Saghafian, A.; Aydogan, M.N.; Arslan, N.P. Microbial Lipid Production by Cold-Adapted Oleaginous Yeast Yarrowia lipolytica B9 in Non-Sterile Whey Medium. BiofuelsBioprod. Biorefining 2015, 9. [Google Scholar] [CrossRef]
- Lappa, I.K.; Papadaki, A.; Kachrimanidou, V.; Terpou, A.; Koulougliotis, D.; Eriotou, E.; Kopsahelis, N. Cheese Whey Processing: Integrated Biorefinery Concepts and Emerging Food Applications. Foods 2019, 8, 347. [Google Scholar]
- Zotta, T.; Solieri, L.; Iacumin, L.; Picozzi, C.; Gullo, M. Valorization of Cheese Whey Using Microbial Fermentations. Appl. Microbiol. Biotechnol. 2020, 104, 2749–2764. [Google Scholar]
- Domingues, L.; Guimarães, P.M.R.; Oliveira, C. Metabolic Engineering of Saccharomyces cerevisiae for Lactose/Whey Fermentation. Bioeng. Bugs 2010, 1, 164–171. [Google Scholar]
- Noike, T.; Takabatake, H.; Mizuno, O.; Ohba, M. Inhibition of Hydrogen Fermentation of Organic Wastes by Lactic Acid Bacteria. Proc. Int. J. Hydrog. Energy 2002, 27. [Google Scholar]
- Carota, E.; Crognale, S.; D’Annibale, A.; Gallo, A.M.; Stazi, S.R.; Petruccioli, M. A Sustainable Use of Ricotta Cheese Whey for Microbial Biodiesel Production. Sci. Total Environ. 2017, 584–585. [Google Scholar] [CrossRef]
- Vyas, S.; Chhabra, M. Assessing Oil Accumulation in the Oleaginous Yeast Cystobasidium oligophagum JRC1 Using Dairy Waste Cheese Whey as a Substrate. 3 Biotech 2019, 9. [Google Scholar] [CrossRef]
- Castanha, R.F.; de Morais, L.A.S.; Mariano, A.P.; Monteiro, R.T.R. Comparison of Two Lipid Extraction Methods Produced by Yeast in Cheese Whey. Braz. Arch. Biol. Technol. 2013, 56. [Google Scholar] [CrossRef] [Green Version]
- Arous, F.; Frikha, F.; Triantaphyllidou, I.E.; Aggelis, G.; Nasri, M.; Mechichi, T. Potential Utilization of Agro-Industrial Wastewaters for Lipid Production by the Oleaginous Yeast Debaryomyces etchellsii. J. Clean. Prod. 2016, 133, 899–909. [Google Scholar] [CrossRef]
- Pirozzi, D.; Ausiello, A.; Zuccaro, G.; Sannino, F.; Yousuf, A. Culture of oleaginous yeasts in dairy industry wastewaters to obtain lipids suitable for the production of II-generation biodiesel. Proc. World Acad. Sci. Eng. Technol. 2013, 8, 57–61. [Google Scholar]
- Arous, F.; Atitallah, I.b.; Nasri, M.; Mechichi, T. A Sustainable Use of Low-Cost Raw Substrates for Biodiesel Production by the Oleaginous Yeast Wickerhamomyces anomalus. 3 Biotech 2017, 7. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food Waste as a Valuable Resource for the Production of Chemicals, Materials and Fuels. Current Situation and Global Perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar]
- Dahiya, S.; Joseph, J. High Rate Biomethanation Technology for Solid Waste Management and Rapid Biogas Production: An Emphasis on Reactor Design Parameters. Bioresour. Technol. 2015, 188. [Google Scholar] [CrossRef]
- Krishna, D.; Kalamdhad, A.S. Pre-Treatment and Anaerobic Digestion of Food Waste for High Rate Methane Production-A Review. J. Environ. Chem. Eng. 2014, 2. [Google Scholar]
- Hafid, H.S.; Rahman, N.A.; Md Shah, U.K.; Baharudin, A.S. Enhanced Fermentable Sugar Production from Kitchen Waste Using Various Pretreatments. J. Environ. Manag. 2015, 156. [Google Scholar] [CrossRef]
- Jiang, J.; Gong, C.; Wang, J.; Tian, S.; Zhang, Y. Effects of Ultrasound Pre-Treatment on the Amount of Dissolved Organic Matter Extracted from Food Waste. Bioresour. Technol. 2014, 155. [Google Scholar] [CrossRef]
- Sarkar, O.; Kumar, A.N.; Dahiya, S.; Krishna, K.V.; Yeruva, D.K.; Mohan, S.V. Regulation of Acidogenic Metabolism towards Enhanced Short Chain Fatty Acid Biosynthesis from Waste: Metagenomic Profiling. RSC Adv. 2016, 6. [Google Scholar] [CrossRef]
- Magdalena, J.A.; Tomás-Pejó, E.; Ballesteros, M.; González-Fernandez, C. Volatile Fatty Acids Production from Protease Pretreated Chlorella Biomass via Anaerobic Digestion. Biotechnol. Prog. 2018, 34. [Google Scholar] [CrossRef]
- Fei, Q.; Chang, H.N.; Shang, L.; dal rae Choi, J.; Kim, N.J.; Kang, J.W. The Effect of Volatile Fatty Acids as a Sole Carbon Source on Lipid Accumulation by Cryptococcus albidus for Biodiesel Production. Bioresour. Technol. 2011, 102. [Google Scholar] [CrossRef]
- Vajpeyi, S.; Chandran, K. Microbial Conversion of Synthetic and Food Waste-Derived Volatile Fatty Acids to Lipids. Bioresour. Technol. 2015, 188. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Zhou, X.; Chen, L.; Zhang, X.; Xin, F.; Dong, W.; Zhang, W.; Ochsenreither, K.; Jiang, M. Bioconversion of Volatile Fatty Acids into Lipids by the Oleaginous Yeast Apiotrichum porosum DSM27194. Fuel 2021, 290. [Google Scholar] [CrossRef]
- Llamas, M.; Dourou, M.; González-Fernández, C.; Aggelis, G.; Tomás-Pejó, E. Screening of Oleaginous Yeasts for Lipid Production Using Volatile Fatty Acids as Substrate. Biomass Bioenergy 2020, 138. [Google Scholar] [CrossRef]
- Annamalai, N.; Sivakumar, N.; Fernandez-Castane, A.; Oleskowicz-Popiel, P. Production of Microbial Lipids Utilizing Volatile Fatty Acids Derived from Wastepaper: A Biorefinery Approach for Biodiesel Production. Fuel 2020, 276. [Google Scholar] [CrossRef]
- Gao, R.; Li, Z.; Zhou, X.; Bao, W.; Cheng, S.; Zheng, L. Enhanced Lipid Production by Yarrowia lipolytica Cultured with Synthetic and Waste-Derived High-Content Volatile Fatty Acids under Alkaline Conditions. Biotechnol. Biofuels 2020, 13. [Google Scholar] [CrossRef]
- Carmona-Cabello, M.; García, I.L.; Papadaki, A.; Tsouko, E.; Koutinas, A.; Dorado, M.P. Biodiesel Production Using Microbial Lipids Derived from Food Waste Discarded by Catering Services. Bioresour. Technol. 2021, 323. [Google Scholar] [CrossRef]
- Ma, X.; Gao, Z.; Gao, M.; Ma, Y.; Ma, H.; Zhang, M.; Liu, Y.; Wang, Q. Microbial Lipid Production from Food Waste Saccharified Liquid and the Effects of Compositions. Energy Convers. Manag. 2018, 172. [Google Scholar] [CrossRef]
- Zeng, Y.; Bian, D.; Xie, Y.; Jiang, X.; Li, X.; Li, P.; Zhang, Y.; Xie, T. Utilization of Food Waste Hydrolysate for Microbial Lipid and Protein Production by Rhodosporidium toruloides Y2. J. Chem. Technol. Biotechnol. 2017, 92. [Google Scholar] [CrossRef]
- Awad, D.; Bohnen, F.; Mehlmer, N.; Brueck, T. Multi-Factorial-Guided Media Optimization for Enhanced Biomass and Lipid Formation by the Oleaginous Yeast Cutaneotrichosporon oleaginosus. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Chopra, J.; Sen, R. Process Optimization Involving Critical Evaluation of Oxygen Transfer, Oxygen Uptake and Nitrogen Limitation for Enhanced Biomass and Lipid Production by Oleaginous Yeast for Biofuel Application. Bioprocess Biosyst. Eng. 2018, 41. [Google Scholar] [CrossRef]
- Abeln, F.; Chuck, C.J. The Role of Temperature, PH and Nutrition in Process Development of the Unique Oleaginous Yeast Metschnikowia pulcherrima. J. Chem. Technol. Biotechnol. 2020, 95. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Chatzifragkou, A.; Kopsahelis, N.; Papanikolaou, S.; Kookos, I.K. Design and Techno-Economic Evaluation of Microbial Oil Production as a Renewable Resource for Biodiesel and Oleochemical Production. Fuel 2014, 116, 566–577. [Google Scholar] [CrossRef]
- Ivančić Šantek, M.; Miškulin, E.; Petrović, M.; Beluhan, S.; Šantek, B. Effect of Carbon and Nitrogen Source Concentrations on the Growth and Lipid Accumulation of Yeast Trichosporon oleaginosus in Continuous and Batch Culture. J. Chem. Technol. Biotechnol. 2017, 92. [Google Scholar] [CrossRef]
- Amaretti, A.; Raimondi, S.; Sala, M.; Roncaglia, L.; de Lucia, M.; Leonardi, A.; Rossi, M. Single Cell Oils of the Cold-Adapted Oleaginous Yeast Rhodotorula glacialis DBVPG 4785. Microb. Cell Factories 2010, 9. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Wu, S.; Wang, Q.; Jin, G.; Shen, H.; Zhao, Z.K. Simultaneous Utilization of Glucose and Xylose for Lipid Production by Trichosporon cutaneum. Biotechnol. Biofuels 2011, 4. [Google Scholar] [CrossRef] [Green Version]
- Wild, R.; Patil, S.; Popović, M.; Zappi, M.; Dufreche, S.; Bajpai, R. Lipids from Lipomyces starkeyi. Food Technol. Biotechnol. 2010, 48. [Google Scholar]
- Tchakouteu, S.S.; Chatzifragkou, A.; Kalantzi, O.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Oleaginous Yeast Cryptococcus curvatus Exhibits Interplay between Biosynthesis of Intracellular Sugars and Lipids. Eur. J. Lipid Sci. Technol. 2015, 117. [Google Scholar] [CrossRef]
- Babazadeh, R.; Lahtvee, P.J.; Adiels, C.B.; Goksör, M.; Nielsen, J.B.; Hohmann, S. The Yeast Osmostress Response Is Carbon Source Dependent. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Dobrowolski, A.; Mituła, P.; Rymowicz, W.; Mirończuk, A.M. Efficient Conversion of Crude Glycerol from Various Industrial Wastes into Single Cell Oil by Yeast Yarrowia lipolytica. Bioresour. Technol. 2016, 207. [Google Scholar] [CrossRef]
- Ratledge, C. Yeasts, moulds, algae and bacteria as sources of lipids. In Technological Advances in Improved and Alternative Sources of Lipids; Springer: Boston, MA, USA, 1994; pp. 235–291. [Google Scholar]
- Tkáčová, J.; Čaplová, J.; Klempová, T.; Čertík, M. Correlation between Lipid and Carotenoid Synthesis in Torularhodin-Producing Rhodotorula glutinis. Ann. Microbiol. 2017, 67. [Google Scholar] [CrossRef]
- Braunwald, T.; Schwemmlein, L.; Graeff-Hönninger, S.; French, W.T.; Hernandez, R.; Holmes, W.E.; Claupein, W. Effect of Different C/N Ratios on Carotenoid and Lipid Production by Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 2013, 97. [Google Scholar] [CrossRef]
- Gong, Z.; Shen, H.; Wang, Q.; Yang, X.; Xie, H.; Zhao, Z.K. Efficient Conversion of Biomass into Lipids by Using the Simultaneous Saccharification and Enhanced Lipid Production Process. Biotechnol. Biofuels 2013, 6. [Google Scholar] [CrossRef] [Green Version]
- Lamers, D.; van Biezen, N.; Martens, D.; Peters, L.; van de Zilver, E.; Jacobs-van Dreumel, N.; Wijffels, R.H.; Lokman, C. Selection of Oleaginous Yeasts for Fatty Acid Production. Bmc Biotechnol. 2016, 16. [Google Scholar] [CrossRef] [Green Version]
- Koganti, S.; Kuo, T.M.; Kurtzman, C.P.; Smith, N.; Ju, L.K. Production of Arabitol from Glycerol: Strain Screening and Study of Factors Affecting Production Yield. Appl. Microbiol. Biotechnol. 2011, 90. [Google Scholar] [CrossRef]
- Nozaki, H.; Suzuki, S.I.; Tsuyoshi, N.; Yokozeki, K. Production of D-Arabitol by Metschnikowia reukaufii AJ14787. Biosci. Biotechnol. Biochem. 2003, 67. [Google Scholar] [CrossRef] [Green Version]
- Papanikolaou, S.; Chevalot, I.; Komaitis, M.; Marc, I.; Aggelis, G. Single Cell Oil Production by Yarrowia lipolytica Growing on an Industrial Derivative of Animal Fat in Batch Cultures. Appl. Microbiol. Biotechnol. 2002, 58. [Google Scholar] [CrossRef]
- Timoumi, A.; Cléret, M.; Bideaux, C.; Guillouet, S.E.; Allouche, Y.; Molina-Jouve, C.; Fillaudeau, L.; Gorret, N. Dynamic Behavior of Yarrowia lipolytica in Response to PH Perturbations: Dependence of the Stress Response on the Culture Mode. Appl. Microbiol. Biotechnol. 2017, 101. [Google Scholar] [CrossRef]
- Kuttiraja, M.; Dhouha, A.; Tyagi, R.D. Harnessing the Effect of PH on Lipid Production in Batch Cultures of Yarrowia lipolytica SKY7. Appl. Biochem. Biotechnol. 2018, 184. [Google Scholar] [CrossRef]
- Slininger, P.J.; Dien, B.S.; Kurtzman, C.P.; Moser, B.R.; Bakota, E.L.; Thompson, S.R.; O’Bryan, P.J.; Cotta, M.A.; Balan, V.; Jin, M.; et al. Comparative Lipid Production by Oleaginous Yeasts in Hydrolyzates of Lignocellulosic Biomass and Process Strategy for High Titers. Biotechnol. Bioeng. 2016, 113, 1676–1690. [Google Scholar] [CrossRef] [Green Version]
- Kot, A.M.; Błażejak, S.; Kurcz, A.; Bryś, J.; Gientka, I.; Bzducha-Wróbel, A.; Maliszewska, M.; Reczek, L. Effect of Initial PH of Medium with Potato Wastewater and Glycerol on Protein, Lipid and Carotenoid Biosynthesis by Rhodotorula glutinis. Electron. J. Biotechnol. 2017, 27. [Google Scholar] [CrossRef]
- Davies, R.J.; Holdsworth, J.E.; Reader, S.L. The Effect of Low Oxygen Uptake Rate on the Fatty Acid Profile of the Oleaginous Yeast Apiotrichum curvatum. Appl. Microbiol. Biotechnol. 1990, 33. [Google Scholar] [CrossRef]
- Naganuma, T.; Uzuka, Y.; Tanaka, K. Physiological Factors Affecting Total Cell Number and Lipid Content of the Yeast, Lipomyces starkeyi. J. Gen. Appl. Microbiol. 1985, 31. [Google Scholar] [CrossRef]
- Calvey, C.H.; Su, Y.K.; Willis, L.B.; McGee, M.; Jeffries, T.W. Nitrogen Limitation, Oxygen Limitation, and Lipid Accumulation in Lipomyces starkeyi. Bioresour. Technol. 2016, 200. [Google Scholar] [CrossRef] [Green Version]
- Yen, H.W.; Zhang, Z. Effects of Dissolved Oxygen Level on Cell Growth and Total Lipid Accumulation in the Cultivation of Rhodotorula glutinis. J. Biosci. Bioeng. 2011, 112. [Google Scholar] [CrossRef]
- Yen, H.W.; Liu, Y.X. Application of Airlift Bioreactor for the Cultivation of Aerobic Oleaginous Yeast Rhodotorula glutinis with Different Aeration Rates. J. Biosci. Bioeng. 2014, 118. [Google Scholar] [CrossRef]
- Saenge, C.; Cheirsilp, B.; Suksaroge, T.T.; Bourtoom, T. Potential Use of Oleaginous Red Yeast Rhodotorula glutinis for the Bioconversion of Crude Glycerol from Biodiesel Plant to Lipids and Carotenoids. Process Biochem. 2011, 46. [Google Scholar] [CrossRef]
- Shuib, S.; Wan Nawi, W.N.N.; Taha, E.M.; Omar, O.; Abdul Kader, A.J.; Kalil, M.S.; Abdul Hamid, A. Strategic Feeding of Ammonium and Metal Ions for Enhanced GLA-Rich Lipid Accumulation in Cunninghamella bainieri 2a1. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Zhao, X.; Kong, X.; Hua, Y.; Feng, B.; Zhao, Z. Medium Optimization for Lipid Production through Co-Fermentation of Glucose and Xylose by the Oleaginous Yeast Lipomyces starkeyi. Eur. J. Lipid Sci. Technol. 2008, 110. [Google Scholar] [CrossRef]
- Saran, S.; Mathur, A.; Dalal, J.; Saxena, R.K. Process Optimization for Cultivation and Oil Accumulation in an Oleaginous Yeast Rhodosporidium toruloides A29. Fuel 2017, 188. [Google Scholar] [CrossRef]
- Kraisintu, P.; Yongmanitchai, W.; Limtong, S. Selection and Optimization for Lipid Production of a Newly Isolated Oleaginous Yeast, Rhodosporidium toruloides DMKU3-TK16. Kasetsart J. Nat. Sci. 2010, 44, 436–445. [Google Scholar]
- Nakagawa, Y.; Sakumoto, N.; Kaneko, Y.; Harashima, S. Mga2p Is a Putative Sensor for Low Temperature and Oxygen to Induce OLE1 Transcription in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2002, 291. [Google Scholar] [CrossRef]
- Tezaki, S.; Iwama, R.; Kobayashi, S.; Shiwa, Y.; Yoshikawa, H.; Ohta, A.; Horiuchi, H.; Fukuda, R. Δ12-Fatty Acid Desaturase Is Involved in Growth at Low Temperature in Yeast Yarrowia lipolytica. Biochem. Biophys. Res. Commun. 2017, 488. [Google Scholar] [CrossRef]
- Liu, Z.J.; Liu, L.P.; Wen, P.; Li, N.; Zong, M.H.; Wu, H. Effects of Acetic Acid and PH on the Growth and Lipid Accumulation of the Oleaginous Yeast Trichosporon fermentans. BioResources 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Gu Pan, J.; Shick Rhee, J. Kinetic and Energetic Analyses of Lipid Accumulation in Batch Culture of Rhodotorula glutinis. J. Ferment. Technol. 1986, 64. [Google Scholar] [CrossRef]
- Issariyakul, T.; Dalai, A.K. Biodiesel from Vegetable Oils. Renew. Sustain. Energy Rev. 2014, 31, 446–471. [Google Scholar]
- Fassinou, W.F.; Sako, A.; Fofana, A.; Koua, K.B.; Toure, S. Fatty Acids Composition as a Means to Estimate the High Heating Value (HHV) of Vegetable Oils and Biodiesel Fuels. Energy 2010, 35. [Google Scholar] [CrossRef]
- Heikal, E.K.; Elmelawy, M.S.; Khalil, S.A.; Elbasuny, N.M. Manufacturing of Environment Friendly Biolubricants from Vegetable Oils. Egypt. J. Pet. 2017, 26. [Google Scholar] [CrossRef] [Green Version]
- Saenge, C.; Cheirsilp, B.; Suksaroge, T.T.; Bourtoom, T. Efficient Concomitant Production of Lipids and Carotenoids by Oleaginous Red Yeast Rhodotorula glutinis Cultured in Palm Oil Mill Effluent and Application of Lipids for Biodiesel Production. Biotechnol. Bioprocess Eng. 2011, 16. [Google Scholar] [CrossRef]
- Kaphueakngam, P.; Flood, A.; Sonwai, S. Production of Cocoa Butter Equivalent from Mango Seed Almond Fat and Palm Oil Mid-Fraction. J. Food Agro-Ind. 2009, 2, 441–447. [Google Scholar]
- Ward, O.P.; Singh, A. Omega-3/6 Fatty Acids: Alternative Sources of Production. Process Biochem. 2005, 40. [Google Scholar] [CrossRef]
- Kothri, M.; Mavrommati, M.; Elazzazy, A.M.; Baeshen, M.N.; Moussa, T.A.A.; Aggelis, G. Microbial Sources of Polyunsaturated Fatty Acids (PUFAs) and the Prospect of Organic Residues and Wastes as Growth Media for PUFA-Producing Microorganisms. FEMS Microbiol. Lett. 2020, 367, 367. [Google Scholar]
- Uemura, H. Synthesis and Production of Unsaturated and Polyunsaturated Fatty Acids in Yeast: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2012, 95, 1–12. [Google Scholar]
- Zhu, Q.; Xue, Z.; Yadav, N.; Damude, H.; Pollak, D.W.; Rupert, R.; Seip, J.; Hollerbach, D.; Macool, D.; Zhang, H.; et al. Metabolic Engineering of an Oleaginous Yeast for the Production of Omega-3 Fatty Acids. In Single Cell Oils: Microbial and Algal Oils, 2nd ed.; AOCS Press: Urbana, IL, USA, 2010; pp. 51–73. [Google Scholar]
- Bellou, S.; Triantaphyllidou, I.E.; Aggeli, D.; Elazzazy, A.M.; Baeshen, M.N.; Aggelis, G. Microbial Oils as Food Additives: Recent Approaches for Improving Microbial Oil Production and Its Polyunsaturated Fatty Acid Content. Curr. Opin. Biotechnol. 2016, 37, 24–35. [Google Scholar]
- Laoteng, K.; Čertík, M.; Cheevadhanark, S. Mechanisms Controlling Lipid Accumulation and Polyunsaturated Fatty Acid Synthesis in Oleaginous Fungi. Chem. Pap. 2011, 65, 97–103. [Google Scholar]
- Béligon, V.; Christophe, G.; Fontanille, P.; Larroche, C. Microbial Lipids as Potential Source to Food Supplements. Curr. Opin. Food Sci. 2016, 7, 35–42. [Google Scholar]
- Fichtali, J.; Senanayake, S.P.J.N. Development and Commercialization of Microalgae-Based Functional Lipids. In Functional Food Product Development; Wiley-Blackwell: Oxford, UK, 2010; Volume 2, pp. 206–225. [Google Scholar]
- Madani, M.; Enshaeieh, M.; Abdoli, A. Single Cell Oil and Its Application for Biodiesel Production. Process Saf. Environ. Prot. 2017, 111, 747–756. [Google Scholar]
- Martinez-Silveira, A.; Villarreal, R.; Garmendia, G.; Rufo, C.; Vero, S. Process Conditions for a Rapid in Situ Transesterification for Biodiesel Production from Oleaginous Yeasts. Electron. J. Biotechnol. 2019, 38. [Google Scholar] [CrossRef]
- Xue, F.; Gao, B.; Zhu, Y.; Zhang, X.; Feng, W.; Tan, T. Pilot-Scale Production of Microbial Lipid Using Starch Wastewater as Raw Material. Bioresour. Technol. 2010, 101. [Google Scholar] [CrossRef]
- Carota, E.; Petruccioli, M.; D’Annibale, A.; Gallo, A.M.; Crognale, S. Orange Peel Waste–Based Liquid Medium for Biodiesel Production by Oleaginous Yeasts. Appl. Microbiol. Biotechnol. 2020, 104. [Google Scholar] [CrossRef]
- Annamalai, N.; Sivakumar, N.; Oleskowicz-Popiel, P. Enhanced Production of Microbial Lipids from Waste Office Paper by the Oleaginous Yeast Cryptococcus curvatus. Fuel 2018, 217. [Google Scholar] [CrossRef]
- Kruger, J.S.; Cleveland, N.S.; Yeap, R.Y.; Dong, T.; Ramirez, K.J.; Nagle, N.J.; Lowell, A.C.; Beckham, G.T.; McMillan, J.D.; Biddy, M.J. Recovery of Fuel-Precursor Lipids from Oleaginous Yeast. ACS Sustain. Chem. Eng. 2018, 6. [Google Scholar] [CrossRef]
- Brenna, E.; Colombo, D.; di Lecce, G.; Gatti, F.G.; Ghezzi, M.C.; Tentori, F.; Tessaro, D.; Viola, M. Conversion of Oleic Acid into Azelaic and Pelargonic Acid by a Chemo-Enzymatic Route. Molecules 2020, 25, 1882. [Google Scholar] [CrossRef]
- Hosney, H.; Nadiem, B.; Ashour, I.; Mustafa, I.; El-Shibiny, A. Epoxidized Vegetable Oil and Bio-Based Materials as PVC Plasticizer. J. Appl. Polym. Sci. 2018, 135, 46270. [Google Scholar] [CrossRef] [Green Version]
- Perez-Sena, W.Y.; Wärnå, J.; Eränen, K.; Tolvanen, P.; Estel, L.; Leveneur, S.; Salmi, T. Use of Semibatch Reactor Technology for the Investigation of Reaction Mechanism and Kinetics: Heterogeneously Catalyzed Epoxidation of Fatty Acid Esters. Chem. Eng. Sci. 2021, 230. [Google Scholar] [CrossRef]
- Munkajohnpong, P.; Kesornpun, C.; Buttranon, S.; Jaroensuk, J.; Weeranoppanant, N.; Chaiyen, P. Fatty Alcohol Production: An Opportunity of Bioprocess. Biofuels Bioprod. Biorefining 2020, 14, 986–1009. [Google Scholar] [CrossRef]
- Owuna, F.J.; Dabai, M.U.; Sokoto, M.A.; Dangoggo, S.M.; Bagudo, B.U.; Birnin-Yauri, U.A.; Hassan, L.G.; Sada, I.; Abubakar, A.L.; Jibrin, M.S. Chemical Modification of Vegetable Oils for the Production of Biolubricants Using Trimethylolpropane: A Review. Egypt. J. Pet. 2020, 29, 75–82. [Google Scholar]

| Species | Organisms | Substrates | Lipid Contents % (w/w) | References |
|---|---|---|---|---|
| Microalgae | Chlorella sp. | - | 53.5 | [24] |
| Botryococcus braunii | - | 34.6 | [25] | |
| Monoraphidium sp | - | 49.6 | [26] | |
| Scenedesmus obliqus | - | 18.5 | [27] | |
| Bacteria | Rhodococcus opacus | molasses | 30.0 | [28] |
| Bacillus subtilis | cotton stalk | 39.8 | [10] | |
| Streptomyces | cellobiose | 47.0 | [9] | |
| Acinetobacter baylyi | glucose | 61.0 | [29] | |
| Fungi | Mortariella isabellina | glucose | 61.0 | [30] |
| Cunninghamella echinulata | glucose | 51.3 | [31] | |
| Mucor moelleri | glycerol | 24.0 | [19] | |
| Aspergillus tubingensis | orange peel waste | 16.0 | [19] | |
| Yeasts | Yarrowia lipolitica | glucose | 45.0 | [32] |
| Lipomyces tetrasporus | cardoon hydrolysate | 47.0 | [23] | |
| Rhodosporidium toruloides | sugarcane molasses | 61.0 | [33] | |
| Lipomyces starkeiy | Arundo donax L | 30.0 | [34] | |
| Trichosporon oleaginous | glucose | 54.0 | [35] |
| Yeasts | Feedstock | Pretreatment | C Source gL−1 | Inhibitors gL−1 | Y Biomass% (w/w) | Y Lipid% (w/w) | Lipid Content % (w/w) | References |
|---|---|---|---|---|---|---|---|---|
| Candida albicans |
Bagasse sugarcane | Steam explosion | reducing sugar 61.33 | nd | 31.8 | 10. | 31.5 | [125] |
| Cryptococcus albidus ATCC 10672 | Sorghum stalks | Diluted alkali |
Glucose 51.0 Xylose 30.0 Arabinose 2.9 |
Acetate 0.5 | 13.2 | 17.0 | 42.0 | [126] |
| Geotrichum candidum NBT-1 | Rice straw | Microwave assisted alkali | Glucose 22.0 Xylose 2.1 Galactose 17.0 | nd | 30.5 | 10.5 | 34.4 | [127] |
|
Yarrowia
lipolytica ATCC 20460 | Wheat straw | Dilute acid |
Glucose 3.7 Xylose 19.6 Arabinose 4.7 Galactose 1.2 | Acetate 4.0 5-HMF 0.1 Fur 0.4 | 26.7 | 1.4 | 4.6 | [99] |
| Lipomyces starkeyi NRRL Y-1389 | Wheat straw | Hydrothermal | Glucose 43.6 Xylose 12.3 | Det | 22.5 | 5.4 | 25.7 | [21] |
|
Lipomyces
starkeyi ATCC 12659 | Wheat straw | Dilute acid |
Glucose 3.7 Xylose 19.6 Arabinose 4.7 Galactose 1.2 | Acetate 4.0 5-HMF 0.1 Fur 0.4 | 50.3 | 15.8 | 31.2 | [99] |
| Lipomyces Starkeyi ATCC 56304 | Sorghum stalks | Diluted alkali |
Glucose 51.0 Xylose 30.0 Arabinose 2.9 | Acetate 0.5 | 21.5 | 16.0 | 44.0 | [126] |
| Lipomyces Starkeyi ATCC 56305 | Switchgrass | Diluted alkali |
Glucose 58.0 Xylose 26.0 | Acetate 0.5 | 19.8 | 17.0 | 39.0 | [126] |
| Lipomyces tetrasporus | Douglas fir | Sulfite and diluted sulfuric acid | Glucose 8.5 Xylose 5.6 Galactose 4.8 Mannose 18.8 | Acetate 6.7 5-HMF 1.8 Fur 1.4 | 35.5 | 11.4 | 23.9 | [128] |
|
Meyerozyma
guilliermondii | Rice husk | Steam explosion | reducing sugar 63.15 | nd | 10.9 | 4.1 | 36.7 | [125] |
| Pichia Kudriavzevii NBT-13 | Rice straw | Microwave assisted alkali | Glucose 22.0 Xylose 2.1 Galactose 17.0 | nd | 17.6 | 6.7 | 37.5 | [127] |
| Pichia kudriavzevii |
Bagasse sugarcane | Steam explosion | Reducing sugar 61.3 | nd | 32.6 | 10.0 | 30.7 | [125] |
| Pichia kudriavzevii | Rice husk | Steam explosion | Reducing sugar 63.15 | nd | 42.3 | 10.0 | 23.6 | [125] |
| Pichia manshurica |
Bagasse sugarcane | Steam explosion | Reducing sugar 61.3 | nd | 38.1 | 9.0 | 23.6 | [125] |
| Pichia kudriavzevii |
Bagasse sugarcane | Steame xplosion | Reducing sugar 61.3 | nd | 13.2 | 4.0 | 30.4 | [125] |
| Rhodotorula Taiwanensis AM2352 | Corncob hydrolysate | Hydrothermal + diluted acid | Glucose 7.2 Xylose 36.8 | Det | 33.9 | 16.9 | 50.1 | [129] |
|
Rhodotorula
glutinis ATCC 204091 | Wheat straw | Dilute acid |
Glucose 3.7 Xylose 19.6 Arabinose 4.7 Galactose 1.2 | Acetate 4.0 5-HMF 0.1 Fu 0.4 | 47.3 | 11.9 | 25.0 | [99] |
|
Rhodotorula
glutinis ATCC 204091 | Wheat straw | Dilute acid |
Glucose 3.2 Xylose 14.0 Arabinose 3.7 Galactose 0.8 | Det | 54.4 | 11.1 | 20.7 | [125] |
| Rhodosporidiobolus fluvialis DMKU-SP314 | Sugarcane | Alkaline hydrogenperoxide | Glucose 18.6 Xylose 6.2 Glycerol 59.0 | Det | 33.6 | 21.2 | 63.3 | [119] |
| Rhodosporidium toruloides NRRL Y-1091 | Wheat straw | Hydrothermal | Glucose 43.6 Xylose 12.34 | Det | 32.1 | 5.0 | 18.7 | [21] |
| Rhodosporidium toruloides DSMZ 4444 | Corn stover | Dilute sodium hydroxide | Glucose 100.0 Xylose 10.0 | Det | 42.9 | 19.0 | 58.6 | [130] |
|
Rhodosporidium
toruloides ATCC 10788 | Wheat straw | Dilute acid |
Glucose 3.2 Xylose 14.0 Arabinose 3.7 Galactose 0.8 | Det | 45.6 | 11.1 | 24.6 | [125] |
| Rhodotorula mucilaginosa |
Bagasse sugarcane | Steam explosion | Reducing sugar 61.3 | nd | 33.8 | 10.0 | 29.5 | [125] |
| Rhodotorula mucilaginosa | Rice husk | Steam explosion | Reducing sugar 63.1 | nd | 40.9 | 10.0 | 24.4 | [125] |
| Trichosporon Dermatis 32903 | Corn stover | Dilute acid |
Glucose 43.4 Xylose 22.7 Arabinose 3.8 Cellobiose 2.3 | Acetate 2.3 5-HMF 2.6 Fur 1.3 Phenol 2.9 | 43.1 | 10.4 | 24.2 | [131] |
| Trichosporon fermentans | Sweet sorghum | Enzymatic saccharification | Sucrose 27.2 Glucose 6.4 Fructose 6.4 | nd | 57.8 | 6.7 | 11.6 | [132] |
| Trichosporon Oleaginosus ATCC 20509 | Switchgrass | Diluted alkali |
Glucose 58.0 Xylose 26.0 |
Acetate 0.5 | 25.1 | 27.0 | 58.0 | [126] |
| Trichosporon Oleaginosus ATCC 20509 | Wheat straw | Dilute acid |
Glucose 3.2 Xylose 14.0 Arabinose 3.7 Galactose 0.8 | Det | 71.9 | 19.4 | 27.1 | [99] |
| Yeasts | OMW Composition (gL−1) | Organic Load Reduction (%, w/w) | Products (gL−1) | References |
|---|---|---|---|---|
| Candida tropicalis ATCC 750 | COD 51.1 * Phenol 2.6 Sugars 13.2 | COD reduction 68 Phenol reduction 39 | Proteasi Lipasi Lipid content 78.7 ** | [148] |
| Candida tropicalis LFMB 16 | Phenol 1.5 Reducing sugars 7.0 + Commercial glucose 65.0 | Decolorization of 16 Phenol reduction 58 | Biomass 2.6 Ethanol 21.9 no lipids production | [147] |
| Cryptococcus curvatus ATCC 20509 | Phenol 1.9 + Commercial xylose 100.0 | Decolorization of 25 Phenol reduction 28 | Biomass 23.8 Lipids 2.5 Lipid content 10.5 ** | [149] |
| Lipomyces starkeyi DSM 702096 | Phenol 1.9 + Commercial xylose 100.0 | No decolorization Phenol reduction 28 | Biomass 21.1 Lipids 5.9 Lipid content 27.9 ** | [149] |
| Lipomyces starkeyi DSM 702096 | Phenol 9.1 Reducing sugars 12.8 | Phenol reduction 43 | Lipid yield 22.4 ** | [150] |
| Rhodotorula mucilaginosa CH4 | COD range 11.6–24.6 * | COD reduction 95.7–56.7 Phenol reduction83–45 | Biomass 9.6 Lipids nd | [151] |
| Yarrowia lipolytica | Phenol 1.5 | Decolorization of 63 Phenol reduction 34 | Biomass 4.8 Lipid content 17.0 ** Citric acid 7.8 | [146] |
| Yarrowia lipolytica A6 | Phenol 1.9 Reducing sugars 7.0 | Phenol reduction 16 | Biomass 2.2 Lipid content 19.1* * | [147] |
| Yarrowia lipolytica A6 | Phenol 1.9 Reducing sugars 7.0 + Glycerol 50.0 | Phenol reduction 16 No uptake of OMW sugars | Biomass 5.6 Lipid content 14.9 ** Mannitol 13.4 | [147] |
| Yarrowia lipolytica LGAM S | Phenol 2.0 Reducing sugars 7.0 | No phenols reduction | Biomass 5.2 Lipid content 6.3 ** Citric acid 6.4 | [147] |
| Yeasts | Treatment | CW Composition (gL−1) | Products (gL−1) | FA | References |
|---|---|---|---|---|---|
| Cystobasidium oligophagum JRC1 | De | COD 66.4 * Reducing sugars 39.6 | Biomass 12.8 Lipids 5.6L ipid content 44.1 ** | 21% C16:0–5% C18:0 45% C18:1–29% C18:2 | [161] |
| Cystobasidium oligophagum JRC1 | NDe | COD 85.5 * Reducing sugars 56.5 | Biomass 20.9 Lipids 4.6 Lipid content 21.8 ** | 5% C14:0–30% C16:0 10% C18:0–40% C18: 115% C18:2 | [161] |
| Cryptoccoccus laurentii- 11 | De | - | Biomass 4.6 Lipids 0.6 Lipid content 13.9 ** | 0.4% C14:0–0.3% C15:0 20.1% C16:0–0. 6% C17:0 27.5% C18:0–34.4% C18:1 4.8% C18:2–1.2% C20:0 0.8% C 22:0–4.8% C24:0 | [162] |
| Debaryomyces etchellsii | De | COD 56.2 * Reducing sugars 25.2 | Biomass 2.8 Lipid content 15.9 ** | 27% C16:0–5% C16:1 4% C18:0–53% C18:1 9% C18:2–2% other | [163] |
| Yarrowia lipolytica B9 | De not sterile | Cheese whey + Lactose | Biomass 7.4 Lipids 4.2 Lipid content 58 ** | 16.9% C16:0–18.7% C16:1 8.4% C17:1–56.1% C18:1 | [155] |
| Lipomyces starkeyi | De | BOD 21.1 * COD 50.8 * Lactose 56.0 Lactic acid 0.5 | Biomass 9.2 Lipid content 18.2 ** | 24% C16:0–14.9% C18:0 49.6% C18:1–5.9% C18:2 | [164] |
| Wickerhamomyces anomalus EC 28 | De | COD 56.2 * Reducing sugars 32.0 | Biomass 2.61 Lipid content 24.0 ** | 33% C16:0–5% C16:1 8% C18:0–32% C18:1 18% C18:2–4% C18:3 | [165] |
| Yeasts | Feedstock | Pretreatment | C Source (gL−1) | Products (gL−1) | References |
|---|---|---|---|---|---|
| Apiotrichum porosum DSM27194 | Corn stover | Diluted acid | Glucose 15.0 + VFA (acetic: propionic: butyric acid = 6:1:1) | Biomass 26.5 Lipid content 36.2 * | [175] |
| Apiotrichum porosum DSM27194 | Corn stover | Diluted acid | Glucose 15.0 + VFA (acetic: propionic: butyric acid = 3:1:2) | Biomass 21.9 Lipid content 31.5 * | [175] |
| Cyberlindnera saturnu NRRL-Y-17396 | Food waste | Anaerobic digestion | VFA 10.0 (acetic: propionic: butyric: valeric acid = 16:6.5:12.5:6) | Biomass yield 32.0 * Lipid yield 11.0 * Lipid content 33.9 * | [176] |
| Cutaneotrichosporon curvatum NRRL-Y-1511 | Food waste | Anaerobic digestion | VFA 15.0 (acetic: propionic: butyric: valeric acid = 11:4:8:4) | Biomass yield 35.0 * Lipid yield 13.0 * Lipid content 36.9 * | [176] |
| Yarrowia lipolytica CICC 31596 | Synthetic | - | Acetic acid 70.0 | Biomass 37.1 Lipids 10.1 Lipid content 27.2 * | [178] |
| Yarrowia lipolytica CICC 31596 | Food waste | Anaerobic digestion | VFA (acetic: propionic: butyric acid = 8:3:5) | Biomass 14.6 Lipid 3.2 Lipid content 21.8 * | [178] |
| Yarrowia lipolytica CICC 31596 | Fruit and vegetable waste | Anaerobic digestion | VFA (acetic: propionic: butyric acid = 5:1:14) | Biomass 11.8 Lipid 3.1 Lipid content 26.0 | [178] |
| Yarrowia lipolytica ACA DC 50109 | Food waste | Anaerobic digestion | VFA 5.0 (acetic: propionic: butyric: valeric acid = 8:3:6:3) | Biomass yield 37.0 * Lipid yield 8 * Lipid content 20.1 * | [176] |
| Lipomyces lipofer NRRL-Y-11555 | Food waste | Anaerobic digestion | VFA15.0(acetic: propionic: butyric: valeric acid = 11:4:8:4) | Biomass yield 30.0 * Lipid yield 5 * Lipid content 16.8 * | [176] |
| Rhodosporidium toruloides Y-27012 | Potato peel | Biological hydrolysis | Glucose 80.0 | Biomass 53.9 Lipids 26.7 Catotenoids nq | [179] |
| Rhodosporidium toruloides 2.1389 | Food waste | Enzymatic hydrolysis | Reducing sugar 50.0 Fats 2.4 Acetic acid 0. 5 Lactic acid 4.0 | Biomass 12.1 Lipid 6.4 Lipid content 52.7 | [180] |
| Rhodosporidium toruloides Y2 | Food waste | Diluted acid | Total sugars 36.7 Fats 10.7 | Biomass 24.7 Lipid 7.3 Lipid content 22.9 * | [181] |
| Rhodotorula toruloides NRRL-Y-27012 | Food waste | Anaerobic digestion | VFA 10.0 (acetic: propionic: butyric: valeric acid = 16:6.5:12.5:6) | Biomass yield 25 * Lipid yield 5 * Lipid content 25.7 * | [176] |
| Lipid Origin | Fatty Acids (Relative %) | References | ||||||
|---|---|---|---|---|---|---|---|---|
| C14:0 | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | ||
| Soybean | – | 10.1 | – | 4.3 | 22.3 | 53.7 | 8.1 | [219] |
| Sunflower | – | 5.2 | 0.1 | 3.7 | 33.7 | 56.5 | – | [219] |
| Rapeseed oil | - | 3.0 | – | 1.0 | 64.4 | 23.3 | 8.0 | [220] |
| Corn | – | 11.6 | – | 2.5 | 38.7 | 44.7 | 1.4 | [219] |
| Jatropha | – | 18.5 | – | 2.3 | 49.0 | 29.7 | – | [220] |
| Cocoa butter | - | 23.3 | 0.9 | 24.5 | 28.7 | 3.9 | – | [220] |
| Palm oil | 0.1 | 39.3 | 0.2 | 4.4 | 42.5 | 11.4 | – | [221] |
| C. albidus ATCC 10672 | - | 20.0 | - | 5.0 | 42.0 | 25.0 | 8.0 | [126] |
| C. curvatus ATCC 20509 | - | 25.9 | - | 15.2 | 47.7 | 6.4 | - | [99] |
| Y. lipolytica ATCC 20460 | - | 6.0 | - | 2.0 | 56.0 | 19.9 | - | [99] |
| L. starkeyi ATCC 56304 | - | 23.0 | 9.0 | 3.0 | 62.0 | 2.0 | 1.0 | [126] |
| L. starkeyi ATCC 12659 | - | 36.2 | - | 4.5 | 46.3 | 3.4 | - | [99] |
| L. tetrasporus DSM 70314 | 0.5 | 39.5 | 4.1 | 12.8 | 40.4 | 0.7 | - | [23] |
| R. glutinis AS 2.1389 | 1.0 | 20.4 | 0.8 | 10.3 | 47.8 | 7.3 | 0.8 | [222] |
| R. glutinis ATCC 204091 | - | 23.5 | - | 9.0 | 43.4 | 15.4 | - | [99] |
| R. taiwanensis AM2352 | 16.7 | 24.4 | 1.4 | 2.9 | 46.8 | 6.5 | - | [129] |
| R. toruloides DSMZ 4444 | 1.3 | 25.1 | - | 10.1 | 45.9 | 10.5 | 3.3 | [130] |
| T. oleaginosus ATCC 20509 | 2.0 | 24.0 | 1.0 | 10.0 | 40.0 | 20.0 | 3.0 | [126] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caporusso, A.; Capece, A.; De Bari, I. Oleaginous Yeasts as Cell Factories for the Sustainable Production of Microbial Lipids by the Valorization of Agri-Food Wastes. Fermentation 2021, 7, 50. https://doi.org/10.3390/fermentation7020050
Caporusso A, Capece A, De Bari I. Oleaginous Yeasts as Cell Factories for the Sustainable Production of Microbial Lipids by the Valorization of Agri-Food Wastes. Fermentation. 2021; 7(2):50. https://doi.org/10.3390/fermentation7020050
Chicago/Turabian StyleCaporusso, Antonio, Angela Capece, and Isabella De Bari. 2021. "Oleaginous Yeasts as Cell Factories for the Sustainable Production of Microbial Lipids by the Valorization of Agri-Food Wastes" Fermentation 7, no. 2: 50. https://doi.org/10.3390/fermentation7020050