Tioconazole-Loaded Transethosomal Gel Using Box–Behnken Design for Topical Applications: In Vitro, In Vivo, and Molecular Docking Approaches
Abstract
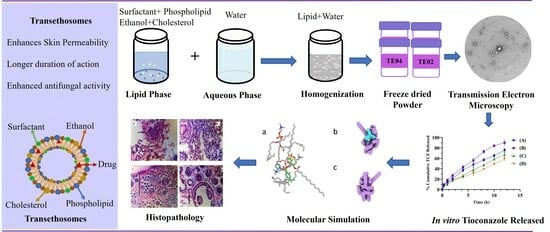
:1. Introduction
2. Results and Discussion
2.1. Box–Behnken Design
2.1.1. Effects of Independent Variables on Vesicle Size (Y1)
2.1.2. Effects of Independent Variables on % Entrapment Efficiency (Y2)
2.1.3. Effects of Independent Variables on % Drug Release (Y3)
2.1.4. Effects of Independent Variables on Zeta Potential (Y4)
2.2. FTIR Studies
2.3. Transmission Electron Microscopy (TEM)
2.4. X-ray Diffraction (XRD) Analysis
2.5. DSC Studies
2.6. Characterization of Fabricated TCZ-Loaded Transethosomal Gels
2.6.1. Physical Appearance, pH, and Conductivity
2.6.2. Drug Content, Spreadability, and Extrudability
2.6.3. Rheological Studies
2.7. In Vitro Drug Release
2.8. Ex Vivo Permeation Studies
2.9. In Vitro Drug Release Kinetics
2.10. In Vitro Antifungal Activity
2.11. Stability Study
2.12. In Vivo Antifungal Activity
2.13. Histopathological Examination
2.14. Molecular Modeling
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Fabrication of TCZ-Loaded TEs
4.3. Box–Behnken Composite Design
4.4. Characterization of Fabricated TCZ-Loaded TEs
4.4.1. Particle Size, Zeta Potential, and Polydispersity Index (PDI)
4.4.2. Fourier Transform Infrared Spectroscopy
4.4.3. % Entrapment Efficiency (% EE)
4.4.4. In Vitro Drug Release
4.4.5. Optical Microscopy
4.4.6. Transmission Electron Microscopy (TEM)
4.4.7. Differential Scanning (DSC)
4.4.8. X-ray Powder Diffraction (XRD)
4.4.9. Storage Stability of Transethosomes
4.5. Preparation of TE Gel
4.5.1. Physical Appearance, Conductivity, and pH Measurement
4.5.2. Extrudability and Spreadability
4.5.3. % Drug Content
4.5.4. Rheological Studies
4.6. Ex Vivo Permeation Studies
4.7. Drug Release Kinetics
4.8. In Vitro Antifungal Activity
4.9. In Vivo Antifungal Activity
4.10. Molecular Modeling
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loh, J.T.; Lam, K.-P. Fungal infections: Immune defense, immunotherapies and vaccines. Adv. Drug Deliv. Rev. 2023, 196, 114775. [Google Scholar] [CrossRef]
- Senoner, T.; Breitkopf, R.; Treml, B.; Rajsic, S. Invasive Fungal Infections after Liver Transplantation. J. Clin. Med. 2023, 12, 3238. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.-Y.; Shi, Y.-J.; Ding, Y.; Lu, W.; Fan, S.-S.; Tao, X.-H. Current status and research progress of nanoparticle application in superficial fungal infection. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 5257–5263. [Google Scholar]
- McCort, M.E.; Tsai, H. Epidemiology of Invasive Candidiasis in Patients with Hematologic Malignancy on Antifungal Prophylaxis. Mycopathologia 2023, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Regidor, P.A.; Thamkhantho, M.; Chayachinda, C.; Palacios, S. Miconazole for the treatment of vulvovaginal candidiasis. In vitro, in vivo and clinical results. Review of the literature. J. Obstet. Gynaecol. 2023, 43, 2195001. [Google Scholar] [CrossRef]
- Moroni, A.B.; Mayoral, E.P.; Lionello, D.F.; Vega, D.R.; Kaufman, T.S.; Calvo, N.L. Characterization of the hydrochloride salt hemihydrate as a new salt of the antifungal agent tioconazole. Int. J. Pharm. 2023, 637, 122869. [Google Scholar] [CrossRef] [PubMed]
- Mali, R.; Patil, J. Nanoparticles: A Novel Antifungal Drug Delivery System. Mater. Proc. 2023, 14, 61. [Google Scholar]
- Song, C.K.; Balakrishnan, P.; Shim, C.-K.; Chung, S.-J.; Chong, S.; Kim, D.-D. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: Characterization and in vitro/in vivo evaluation. Colloids Surf. B Biointerfaces 2012, 92, 299–304. [Google Scholar] [CrossRef]
- Vijeta, B.; Namrata, M.; Alagusundaram, M. Ultra Deformable Nanotransethosomes: A Novel Tool to Intensify Transdermal Drug Delivery a Review. J. Pharm. Negat. Results 2023, 14, 2024–2032. [Google Scholar]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes—Novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Paolino, D.; Lucania, G.; Mardente, D.; Alhaique, F.; Fresta, M. Ethosomes for skin delivery of ammonium glycyrrhizinate: In vitro percutaneous permeation through human skin and in vivo anti-inflammatory activity on human volunteers. J. Control. Release 2005, 106, 99–110. [Google Scholar] [CrossRef]
- Harbi, I.; Aljaeid, B.; El-Say, K.M.; Zidan, A.S. Glycosylated sertraline-loaded liposomes for brain targeting: QbD study of formulation variabilities and brain transport. AAPS PharmSciTech 2016, 17, 1404–1420. [Google Scholar] [CrossRef]
- Ahmed, T.A. Development of rosuvastatin flexible lipid-based nanoparticles: Promising nanocarriers for improving intestinal cells cytotoxicity. BMC Pharmacol. Toxicol. 2020, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Alomrani, A.; Badran, M.; Harisa, G.I.; ALshehry, M.; Alhariri, M.; Alshamsan, A.; Alkholief, M. The use of chitosan-coated flexible liposomes as a remarkable carrier to enhance the antitumor efficacy of 5-fluorouracil against colorectal cancer. Saudi Pharm. J. 2019, 27, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Aqil, M.; Kohli, K.; Sultana, Y.; Mujeeb, M. Enhanced transdermal delivery of an anti-hypertensive agent via nanoethosomes: Statistical optimization, characterization and pharmacokinetic assessment. Int. J. Pharm. 2013, 443, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fan, Y.; Fan, C.; Li, X.; Wang, X.; Li, M.; Liu, Y. Tacrolimus-loaded ethosomes: Physicochemical characterization and in vivo evaluation. Eur. J. Pharm. Biopharm. 2012, 82, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.K.; Abou Assi, R.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279. [Google Scholar] [CrossRef]
- Ahmed, T.A. Preparation of transfersomes encapsulating sildenafil aimed for transdermal drug delivery: Plackett–Burman design and characterization. J. Liposome Res. 2015, 25, 1–10. [Google Scholar] [CrossRef]
- Al-Mahallawi, A.M.; Abdelbary, A.A.; Aburahma, M.H. Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int. J. Pharm. 2015, 485, 329–340. [Google Scholar] [CrossRef]
- Mahmood, S.; Mandal, U.K.; Chatterjee, B. Transdermal delivery of raloxifene HCl via ethosomal system: Formulation, advanced characterizations and pharmacokinetic evaluation. Int. J. Pharm. 2018, 542, 36–46. [Google Scholar] [CrossRef]
- Ogiso, T.; Yamaguchi, T.; Iwaki, M.; Tanino, T.; Miyake, Y. Effect of positively and negatively charged liposomes on skin permeation of drugs. J. Drug Target. 2001, 9, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Crisóstomo-Lucas, C.; García-Holley, P.; Hernández-Ortega, S.; Sánchez-Bartéz, F.; Gracia-Mora, I.; Barba-Behrens, N. Structural characterization and cytotoxic activity of tioconazole coordination compounds with cobalt (II), copper (II), zinc (II) and cadmium (II). Inorganica Chim. Acta 2015, 438, 245–254. [Google Scholar] [CrossRef]
- Shaji, J.E.S.S.Y.; Bhatia, V. Dissolution enhancement of atovaquone through cyclodextrin complexation and phospholipid solid dispersion. Int. J. Pharm. Pharm. Sci. 2013, 5, 642–650. [Google Scholar]
- Zhang, Z.; Wo, Y.; Zhang, Y.; Wang, D.; He, R.; Chen, H.; Cui, D. In vitro study of ethosome penetration in human skin and hypertrophic scar tissue. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chaudhury, A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech 2011, 12, 62–76. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Khalid, M. Transdermal film-loaded finasteride microplates to enhance drug skin permeation: Two-step optimization study. Eur. J. Pharm. Sci. 2016, 88, 246–256. [Google Scholar] [CrossRef]
- Ribeiro, R.F.; Motta, M.H.; Härter, A.P.G.; Flores, F.C.; Beck, R.C.R.; Schaffazick, S.R.; da Silva, C.d.B. Spray-dried powders improve the controlled release of antifungal tioconazole-loaded polymeric nanocapsules compared to with lyophilized products. Mater. Sci. Eng. C 2016, 59, 875–884. [Google Scholar] [CrossRef]
- Yang, J.; Alvebratt, C.; Lu, X.; Bergström, C.A.; Strømme, M.; Welch, K. Amorphous magnesium carbonate nanoparticles with strong stabilizing capability for amorphous ibuprofen. Int. J. Pharm. 2018, 548, 515–521. [Google Scholar] [CrossRef]
- Verma, P.; Pathak, K. Nanosized ethanolic vesicles loaded with econazole nitrate for the treatment of deep fungal infections through topical gel formulation. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 489–496. [Google Scholar] [CrossRef]
- Kumari, S.; Pathak, K. Cavamax W7 composite psoralen ethosomal gel versus cavamax W7 psoralen solid complex gel for topical delivery: A comparative evaluation. Int. J. Pharm. Investig. 2013, 3, 171. [Google Scholar]
- Cheng, X.; McCoy, J.H.; Israelachvili, J.N.; Cohen, I. Imaging the microscopic structure of shear thinning and thickening colloidal suspensions. Science 2011, 333, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.F.; Nafady, M.M.; Kharshoum, R.M.; el-Ghafar, A.; Omnia, A.; Farouk, H.O.J.A.P. Novel enhanced therapeutic efficacy of dapoxetine HCl by nano-vesicle transdermal gel for treatment of carrageenan-induced rat paw edema. AAPS PharmSciTech 2020, 21, 113. [Google Scholar] [CrossRef]
- Singh, S.; Verma, D.; Mirza, M.A.; Das, A.K.; Anwer, M.K.; Sultana, Y.; Talegaonkar, S.; Iqbal, Z. Development and optimization of ketoconazole loaded nano-transfersomal gel for vaginal delivery using Box-Behnken design: In vitro, ex vivo characterization and antimicrobial evaluation. J. Drug Deliv. Sci. Technol. 2017, 39, 95–103. [Google Scholar] [CrossRef]
- Lefler, E.; Stevens, D.A. Inhibition and killing of Candida albicans in vitro by five imidazoles in clinical use. Antimicrob. Agents Chemother. 1984, 25, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, K.R.; Fadaly, W.A. New 1, 2-diaryl-4-substituted-benzylidene-5-4H-imidazolone derivatives: Design, synthesis and biological evaluation as potential anti-inflammatory and analgesic agents. Bioorg. Chem. 2017, 72, 123–129. [Google Scholar] [CrossRef]
- Satyam, S.M.; Bairy, K.L.; Musharaf, S.; Fernandes, D.; Prakash, P.Y. Assessment of Anti-Dermatophytic Activity of Zincoderm gm Cream in Experimental Tinea Pedis in Wistar Rats. Int. J. Pharm. Chem. Biol. Sci. 2015, 5, 683–687. [Google Scholar]
- Qushawy, M.; Nasr, A.; Abd-Alhaseeb, M.; Swidan, S. Design, optimization and characterization of a transfersomal gel using miconazole nitrate for the treatment of candida skin infections. Pharmaceutics 2018, 10, 26. [Google Scholar] [CrossRef]
- Šentjurc, M.; Vrhovnik, K.; Kristl, J. Liposomes as a topical delivery system: The role of size on transport studied by the EPR imaging method. J. Control. Release 1999, 59, 87–97. [Google Scholar] [CrossRef]
- Araújo, J.; Vega, E.; Lopes, C.; Egea, M.; Garcia, M.; Souto, E.B. Effect of polymer viscosity on physicochemical properties and ocular tolerance of FB-loaded PLGA nanospheres. Colloids Surf. B Biointerfaces 2009, 72, 48–56. [Google Scholar] [CrossRef]
- Siddiqui, A.; Jain, P.; Alex, T.S.; Ali, M.A.; Hassan, N.; Haneef, J.; Naseef, P.P.; Kuruniyan, M.S.; Mirza, M.A.; Iqbal, Z. Investigation of a Minocycline-Loaded Nanoemulgel for the Treatment of Acne Rosacea. Pharmaceutics 2022, 14, 2322. [Google Scholar] [CrossRef]
- Attama, A.; Reichl, S.; Müller-Goymann, C.C. Sustained release and permeation of timolol from surface-modified solid lipid nanoparticles through bioengineered human cornea. Curr. Eye Res. 2009, 34, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Bisht, D.; Verma, D.; Mirza, M.A.; Anwer, M.K.; Iqbal, Z. Development of ethosomal gel of ranolazine for improved topical delivery: In vitro and ex vivo evaluation. J. Mol. Liq. 2017, 225, 475–481. [Google Scholar] [CrossRef]
- Agrawal, Y.; Petkar, K.C.; Sawant, K.K. Development, evaluation and clinical studies of Acitretin loaded nanostructured lipid carriers for topical treatment of psoriasis. Int. J. Pharm. 2010, 401, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Karavelidis, V.; Giliopoulos, D.; Karavas, E.; Bikiaris, D. Nanoencapsulation of a water soluble drug in biocompatible polyesters. Effect of polyesters melting point and glass transition temperature on drug release behavior. Eur. J. Pharm. Sci. 2010, 41, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Aggarwal, A.K.; Rechkoblit, O. Eukaryotic DNA polymerases. Curr. Opin. Struct. Biol. 2018, 53, 77–87. [Google Scholar] [CrossRef]
- Dantas, M.G.B.; Reis, S.A.G.B.; Damasceno, C.M.D.; Rolim, L.A.; Rolim-Neto, P.J.; Carvalho, F.O.; Quintans-Junior, L.J.; Almeida, J.R.G.d.S. Development and evaluation of stability of a gel formulation containing the monoterpene borneol. Sci. World J. 2016, 2016, 7394685. [Google Scholar] [CrossRef]
- Jamadar, M.J.; Shaikh, R.H. Preparation and evaluation of herbal gel formulation. J. Pharm. Res. Educ. 2017, 1, 201–224. [Google Scholar]
- Altuntaş, E.; Yener, G. Anti-aging potential of a cream containing herbal oils and honey: Formulation and in vivo evaluation of effectiveness using non-invasive biophysical techniques. IOSR J. Pharm. Biol. Sci. 2015, 10, 51–60. [Google Scholar]
- Thomas, L.; Zakir, F.; Mirza, M.A.; Anwer, M.K.; Ahmad, F.J.; Iqbal, Z. Development of Curcumin loaded chitosan polymer based nanoemulsion gel: In vitro, ex vivo evaluation and in vivo wound healing studies. Int. J. Biol. Macromol. 2017, 101, 569–579. [Google Scholar] [CrossRef]
- Pfaller, M.; Messer, S.; Coffmann, S. Comparison of visual and spectrophotometric methods of MIC endpoint determinations by using broth microdilution methods to test five antifungal agents, including the new triazole D0870. J. Clin. Microbiol. 1995, 33, 1094–1097. [Google Scholar] [CrossRef]











| Independent Variables | Dependent Variables | |||||||
|---|---|---|---|---|---|---|---|---|
| Run | Code | X1 (% w/v) | X2 (% v/v) | X3 (% w/w) | Y1 (nm) | Y2 (%) | Y3 (%) | Y4 mV |
| 01 | TE01 | 3.5 | 30 | 17.5 | 254.4 ± 2.13 | 80.71 ± 0.21 | 85.12 ± 0.54 | −21.2 ± 0.23 |
| 02 | TE02 | 05 | 30 | 10.0 | 314.4 ± 3.21 | 65.12 ± 0.84 | 81.23 ± 0.35 | −25.8 ± 0.34 |
| 03 | TE03 | 3.5 | 40 | 10.0 | 610.3 ± 1.14 | 67.34 ± 0.54 | 90.14 ± 0.45 | −18.2 ± 0.56 |
| 04 | TE04 | 3.5 | 20 | 25.0 | 229.5 ± 2.19 | 86.13 ± 0.46 | 92.03 ± 0.67 | −1.50 ± 0.23 |
| 05 | TE05 | 05 | 20 | 17.5 | 432.1 ± 3.26 | 82.34 ± 0.35 | 90.04 ± 0.41 | −7.19 ± 0.56 |
| 06 | TE06 | 02 | 20 | 17.5 | 402.7 ± 3.98 | 68.24 ± 0.45 | 79.12 ± 0.37 | −13.9 ± 0.21 |
| 07 | TE07 | 02 | 30 | 10.0 | 235.3 ± 2.24 | 63.34 ± 0.97 | 77.01 ± 0.59 | −24.3 ± 0.56 |
| 08 | TE08 | 3.5 | 40 | 25.0 | 757.1 ± 2.54 | 70.35 ± 0.54 | 82.24 ± 0.61 | −15.8 ± 0.43 |
| 09 | TE09 | 3.5 | 20 | 10.0 | 522.8 ± 3.65 | 60.56 ± 0.21 | 85.43 ± 0.54 | −28.5 ± 0.23 |
| 10 | TE10 | 3.5 | 30 | 17.5 | 219.1 ± 4.21 | 63.43 ± 0.56 | 84.02 ± 0.71 | −3.79 ± 0.67 |
| 11 | TE11 | 3.5 | 30 | 17.5 | 219.1 ± 2.45 | 63.43 ± 0.24 | 84.02 ± 0.34 | −3.79 ± 0.98 |
| 12 | TE12 | 05 | 40 | 17.5 | 227.8 ± 3.43 | 73.43 ± 0.56 | 85.21 ± 0.67 | −1.27 ± 0.43 |
| 13 | TE13 | 02 | 30 | 25.0 | 248.5 ± 1.54 | 74.87 ± 0.23 | 81.24 ± 0.23 | −23.8 ± 0.78 |
| 14 | TE14 | 05 | 30 | 25.0 | 378.5 ± 5.23 | 83.39 ± 0.54 | 85.21 ± 0.56 | −14.8 ± 0.23 |
| 15 | TE15 | 02 | 40 | 17.5 | 239.3 ± 2.45 | 69.3 ± 0.35 | 79.29 ± 0.32 | −1.04 ± 0.43 |
| Fit Model | Response | R2 | Adjusted R2 | Predicted R2 | SD | % CV |
|---|---|---|---|---|---|---|
| Quadratic | Vesicle size (Y1) | 0.6547 | 0.0331 | −4.4953 | 162.73 | 46.13 |
| Linear | % EE (Y2) | 0.5842 | 0.4708 | −0.3307 | 6.04 | 8.47 |
| Quadratic | % Drug release (Y3) | 0.9198 | 0.7755 | −0.2474 | 2.05 | 2.44 |
| Quadratic | Zeta potential (Y4) | 0.7749 | 0.3697 | −0.5651 | 7.84 | 57.38 |
| No. | Code | pH | Conductivity (µs/cm) | % Drug Content | Spreadability (g/s) | Extrudability (g/cm2) |
|---|---|---|---|---|---|---|
| 01 | TEG01 | 6.79 ± 0.03 | 913± 0.03 | 98.15 ± 0.21 | 5.28 ± 0.15 | 8.35 ± 0.05 |
| 02 | TEG04 | 6.73 ± 0.01 | 974± 0.02 | 99.24 ± 0.31 | 5.75 ± 0.15 | 8.64 ± 0.01 |
| 03 | TEG06 | 6.61 ± 0.04 | 975± 0.03 | 98.64 ± 0.15 | 5.22 ± 0.15 | 8.53 ± 0.05 |
| 04 | TEG13 | 6.82± 0.03 | 912± 0.01 | 98.86 ± 0.15 | 5.34 ± 0.15 | 8.44 ± 0.07 |
| Formulation Code | Jmax (µg cm−2 h−1) | Kp (cm h−1) | ER |
|---|---|---|---|
| TCZ gel | 6.8 | 0.53 | 5.2 |
| TEG04 | 24.1 | 3.1 |
| Fungal Species | Code | MIC (µg/mL) | MFC (µg/mL) |
|---|---|---|---|
| Aspergillus fumigatus | TCZ | 5.8 ± 0.12 | 34.2 ± 0.14 |
| TE04 | 4.3 ± 0.54 | 21.6 ± 0.25 | |
| TEG04 | 5.3 ± 0.98 | 24.1 ± 0.37 | |
| Candida albicans | TCZ | 2.8 ± 0.23 | 24.4 ± 0.45 |
| TE04 | 1.9 ± 0.34 | 17.2 ± 0.67 | |
| TEG04 | 2.3 ± 0.18 | 19.8 ± 0.21 |
| Time | Drug Content | Particle Size |
|---|---|---|
| Initial | 99.54 ± 0.14 | 229.5 ± 2.34 |
| After 2 months | 98.53 ± 0.32 | 234.5 ± 3.12 |
| After 4 months | 98.10 ± 0.21 | 238.1 ± 4.41 |
| After 6 months | 98.21 ± 0.15 | 240.4 ± 3.64 |
| Time | Drug Content | pH | Homogeneity |
|---|---|---|---|
| Initial | 99.24 ± 0.26 | 6.8 ± 0.25 | Homogeneous |
| After 2 months | 98.53 ± 0.53 | 6.5 ± 0.04 | Homogeneous |
| After 4 months | 98.10 ± 0.64 | 6.9 ± 0.19 | Homogeneous |
| After 6 months | 98.21 ± 0.35 | 7.1 ± 0.31 | Homogeneous |
| Group | Fungal Hyphae | Hyperkeratosis | Acanthosis | Interface Dermatitis | Inflammation | Dermis |
|---|---|---|---|---|---|---|
| Group 1 (Negative control) | 0 | 0 | 0 | 0 | 0 | 0 |
| Group 2 (Positive control) | 1 | 1 | 1 | 1 | 2 | 1 |
| Group 3 (Experimental drug) | 0 | 0 | 0 | 0 | 0 | 0 |
| Group 4 (Market drug) | 0 | 0 | 1 | 0 | 0 | 0 |
| Variables | Level | |
|---|---|---|
| Low | High | |
| Independent variables | ||
| X1: Phospholipids (Lipoid S100) % w/v | 2 | 5 |
| X2: Ethanol % v/v | 20 | 40 |
| X3: Surfactant (Tween 80) % w/w of total Lipoid S100 | 10 | 25 |
| Dependent Variables | ||
| Y1: Vesicle size (nm) | ||
| Y2: % Entrapment Efficiency | ||
| Y3: % Drug release | ||
| Y4: Zeta potential (mV) | ||
| Run | Phospholipids % w/v | Ethanol % v/v | Surfactant % w/w of Total Lipids | Cholesterol % w/v | Drug % w/v |
|---|---|---|---|---|---|
| 01 | 3.5 | 30 | 17.5 | 0.5 | 1 |
| 02 | 05 | 30 | 10.0 | 0.5 | 1 |
| 03 | 3.5 | 40 | 10.0 | 0.5 | 1 |
| 04 | 3.5 | 20 | 25.0 | 0.5 | 1 |
| 05 | 05 | 20 | 17.5 | 0.5 | 1 |
| 06 | 02 | 20 | 17.5 | 0.5 | 1 |
| 07 | 02 | 30 | 10.0 | 0.5 | 1 |
| 08 | 3.5 | 40 | 25.0 | 0.5 | 1 |
| 09 | 3.5 | 20 | 10.0 | 0.5 | 1 |
| 10 | 3.5 | 30 | 17.5 | 0.5 | 1 |
| 11 | 3.5 | 30 | 17.5 | 0.5 | 1 |
| 12 | 05 | 40 | 17.5 | 0.5 | 1 |
| 13 | 02 | 30 | 25.0 | 0.5 | 1 |
| 14 | 05 | 30 | 25.0 | 0.5 | 1 |
| 15 | 02 | 40 | 17.5 | 0.5 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qureshi, M.I.; Jamil, Q.A.; Usman, F.; Wani, T.A.; Farooq, M.; Shah, H.S.; Ahmad, H.; Khalil, R.; Sajjad, M.; Zargar, S.; et al. Tioconazole-Loaded Transethosomal Gel Using Box–Behnken Design for Topical Applications: In Vitro, In Vivo, and Molecular Docking Approaches. Gels 2023, 9, 767. https://doi.org/10.3390/gels9090767
Qureshi MI, Jamil QA, Usman F, Wani TA, Farooq M, Shah HS, Ahmad H, Khalil R, Sajjad M, Zargar S, et al. Tioconazole-Loaded Transethosomal Gel Using Box–Behnken Design for Topical Applications: In Vitro, In Vivo, and Molecular Docking Approaches. Gels. 2023; 9(9):767. https://doi.org/10.3390/gels9090767
Chicago/Turabian StyleQureshi, Muhammad Imran, Qazi Adnan Jamil, Faisal Usman, Tanveer A. Wani, Mudassir Farooq, Hamid Saeed Shah, Hassan Ahmad, Ruqaiya Khalil, Muhammad Sajjad, Seema Zargar, and et al. 2023. "Tioconazole-Loaded Transethosomal Gel Using Box–Behnken Design for Topical Applications: In Vitro, In Vivo, and Molecular Docking Approaches" Gels 9, no. 9: 767. https://doi.org/10.3390/gels9090767