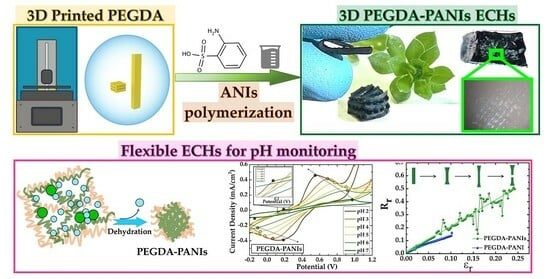
Self-Standing 3D-Printed PEGDA–PANIs Electroconductive Hydrogel Composites for pH Monitoring
Abstract
:1. Introduction
2. Results and Discussion
2.1. PANIs and PANI Characterizations
2.2. PEGDA–PANIs and PEGDA–PANI Composites
2.2.1. Morphological and Structural Characterization
2.2.2. Swelling Degree and Water Retention Analyses
2.2.3. Electrochemical Activity
2.2.4. CVs as a Function of pH
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. 3D Printing of PEGDA Substrates
4.3. Preparation of PANI-Based Reference Samples
4.4. Preparation of PEGDA–PANIs Composites
4.5. Characterization Techniques
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kougkolos, G.; Golzio, M.; Laudebat, L.; Valdez-Nava, Z.; Flahaut, E. Hydrogels with Electrically Conductive Nanomaterials for Biomedical Applications. J. Mater. Chem. B 2023, 11, 2036–2062. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Alvarez-Perez, M.A.; Borriello, A.; Napolitano, T.; Ambrosio, L. Conductive PANi/PEGDA Macroporous Hydrogels For Nerve Regeneration. Adv. Healthc. Mater. 2013, 2, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, Y.X.; Yan, J.; Yang, S.; Dong, P.; Soman, P. Fabrication of Conductive Polyaniline Hydrogel Using Porogen Leaching and Projection Microstereolithography. J. Mater. Chem. B 2015, 3, 5352–5360. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Lee, S.J.; Timsina, R.; Qiu, X.; Castro, N.J.; Zhang, L.G. Development of 3D Printable Conductive Hydrogel with Crystallized PEDOT:PSS for Neural Tissue Engineering. Mater. Sci. Eng. C 2019, 99, 582–590. [Google Scholar] [CrossRef]
- Rogers, Z.J.; Zeevi, M.P.; Koppes, R.; Bencherif, S.A. Electroconductive Hydrogels for Tissue Engineering: Current Status and Future Perspectives. Bioelectricity 2020, 2, 279–292. [Google Scholar] [CrossRef]
- Guo, B.; Zhong, Y.; Chen, X.; Yu, S.; Bai, J. 3D Printing of Electrically Conductive and Degradable Hydrogel for Epidermal Strain Sensor. Compos. Commun. 2023, 37, 101454. [Google Scholar] [CrossRef]
- Athukorala, S.S.; Tran, T.S.; Balu, R.; Truong, V.K.; Chapman, J.; Dutta, N.K.; Roy Choudhury, N. 3D Printable Electrically Conductive Hydrogel Scaffolds for Biomedical Applications: A Review. Polymers 2021, 13, 474. [Google Scholar] [CrossRef]
- Aggas, J.R.; Abasi, S.; Phipps, J.F.; Podstawczyk, D.A.; Guiseppi-Elie, A. Microfabricated and 3-D Printed Electroconductive Hydrogels of PEDOT:PSS and Their Application in Bioelectronics. Biosens. Bioelectron. 2020, 168, 112568. [Google Scholar] [CrossRef]
- Distler, T.; Boccaccini, A.R. 3D Printing of Electrically Conductive Hydrogels for Tissue Engineering and Biosensors—A Review. Acta Biomater. 2020, 101, 1–13. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Kang, W.; Liu, X.; Wang, Q. Current Advances and Future Perspectives of Additive Manufacturing for Functional Polymeric Materials and Devices. SusMat 2021, 1, 127–147. [Google Scholar] [CrossRef]
- Lee, J.Y.; An, J.; Chua, C.K. Fundamentals and Applications of 3D Printing for Novel Materials. Appl. Mater. Today 2017, 7, 120–133. [Google Scholar] [CrossRef]
- Turner, B.N.; Strong, R.; Gold, S.A. A Review of Melt Extrusion Additive Manufacturing Processes: I. Process Design and Modeling. Rapid Prototyp. J. 2014, 20, 192–204. [Google Scholar] [CrossRef]
- Ul Haq, A.; Montaina, L.; Pescosolido, F.; Carotenuto, F.; Trovalusci, F.; De Matteis, F.; Tamburri, E.; Di Nardo, P. Electrically Conductive Scaffolds Mimicking the Hierarchical Structure of Cardiac Myofibers. Sci. Rep. 2023, 13, 2863. [Google Scholar] [CrossRef] [PubMed]
- Politi, S.; Tamburri, E.; Carcione, R.; Lavecchia, T.; Angjellari, M.; Terranova, M.L. Innovative Preparation Processes and Structural Characteristics of 3D Printable Polymer-Based Nanocomposites. In Proceedings of the AIP Conference Proceedings, Ischia, Italy, 12–14 September 2019; Volume 2196. [Google Scholar]
- Wallin, T.J.; Pikul, J.; Shepherd, R.F. 3D Printing of Soft Robotic Systems. Nat. Rev. Mater. 2018, 3, 84–100. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Zhang, N.; Hingorani, H.; Ding, N.; Wang, D.; Yuan, C.; Zhang, B.; Gu, G.; Ge, Q. Fast-Response, Stiffness-Tunable Soft Actuator by Hybrid Multimaterial 3D Printing. Adv. Funct. Mater. 2019, 29, 1806698. [Google Scholar] [CrossRef]
- Truby, R.L.; Wehner, M.; Grosskopf, A.K.; Vogt, D.M.; Uzel, S.G.M.; Wood, R.J.; Lewis, J.A. Soft Somatosensitive Actuators via Embedded 3D Printing. Adv. Mater. 2018, 30, 1706383. [Google Scholar] [CrossRef]
- Foster, C.W.; Elbardisy, H.M.; Down, M.P.; Keefe, E.M.; Smith, G.C.; Banks, C.E. Additively Manufactured Graphitic Electrochemical Sensing Platforms. Chem. Eng. J. 2020, 381, 122343. [Google Scholar] [CrossRef]
- Rim, Y.S.; Bae, S.H.; Chen, H.; De Marco, N.; Yang, Y. Recent Progress in Materials and Devices toward Printable and Flexible Sensors. Adv. Mater. 2016, 28, 4415–4440. [Google Scholar] [CrossRef]
- Murr, L.E. Frontiers of 3D Printing/Additive Manufacturing: From Human Organs to Aircraft Fabrication. J. Mater. Sci. Technol. 2016, 32, 987–995. [Google Scholar] [CrossRef]
- Gisario, A.; Kazarian, M.; Martina, F.; Mehrpouya, M. Metal Additive Manufacturing in the Commercial Aviation Industry: A Review. J. Manuf. Syst. 2019, 53, 124–149. [Google Scholar] [CrossRef]
- Angjellari, M.; Tamburri, E.; Montaina, L.; Natali, M.; Passeri, D.; Rossi, M.; Terranova, M.L. Beyond the Concepts of Nanocomposite and 3D Printing: PVA and Nanodiamonds for Layer-by-Layer Additive Manufacturing. Mater. Des. 2017, 119, 12–21. [Google Scholar] [CrossRef]
- Montaina, L.; Carcione, R.; Pescosolido, F.; Montalto, M.; Battistoni, S.; Tamburri, E. Three-Dimensional-Printed Polyethylene Glycol Diacrylate-Polyaniline Composites by in Situ Aniline Photopolymerization: An Innovative Biomaterial for Electrocardiogram Monitoring Systems. ACS Appl. Electron. Mater. 2022, 5, 164–172. [Google Scholar] [CrossRef]
- Smith, C.F.; Tollemache, N.; Covill, D.; Johnston, M. Take Away Body Parts! An Investigation into the Use of 3D-Printed Anatomical Models in Undergraduate Anatomy Education. Anat. Sci. Educ. 2018, 11, 44–53. [Google Scholar] [CrossRef]
- Zarek, M.; Layani, M.; Cooperstein, I.; Sachyani, E.; Cohn, D.; Magdassi, S. 3D Printing of Shape Memory Polymers for Flexible Electronic Devices. Adv. Mater. 2016, 28, 4449–4454. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Carmone, S.; Brambilla, D.; Leroux, J.C. 3D Printing of a Wearable Personalized Oral Delivery Device: A First-in-Human Study. Sci. Adv. 2018, 4, eaat2544. [Google Scholar] [CrossRef]
- Ma, X.; Liu, J.; Zhu, W.; Tang, M.; Lawrence, N.; Yu, C.; Gou, M.; Chen, S. 3D Bioprinting of Functional Tissue Models for Personalized Drug Screening and in Vitro Disease Modeling. Adv. Drug Deliv. Rev. 2018, 132, 235–251. [Google Scholar] [CrossRef]
- Bose, S.; Ke, D.; Sahasrabudhe, H.; Bandyopadhyay, A. Additive Manufacturing of Biomaterials. Prog. Mater. Sci. 2018, 93, 45–111. [Google Scholar] [CrossRef]
- Valentine, A.D.; Busbee, T.A.; Boley, J.W.; Raney, J.R.; Chortos, A.; Kotikian, A.; Berrigan, J.D.; Durstock, M.F.; Lewis, J.A. Hybrid 3D Printing of Soft Electronics. Adv. Mater. 2017, 29, 1703817. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B. Additive Manufacturing Technologies: 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–498. [Google Scholar]
- Gonzalez, G.; Roppolo, I.; Pirri, C.F.; Chiappone, A. Current and Emerging Trends in Polymeric 3D Printed Microfluidic Devices. Addit. Manuf. 2022, 55, 102867. [Google Scholar] [CrossRef]
- Isreb, A.; Baj, K.; Wojsz, M.; Isreb, M.; Peak, M.; Alhnan, M.A. 3D Printed Oral Theophylline Doses with Innovative ‘Radiator-like’ Design: Impact of Polyethylene Oxide (PEO) Molecular Weight. Int. J. Pharm. 2019, 564, 98–105. [Google Scholar] [CrossRef]
- Scordo, G.; Bertana, V.; Ballesio, A.; Carcione, R.; Marasso, S.L.; Cocuzza, M.; Pirri, C.F.; Manachino, M.; Gomez, M.G.; Vitale, A.; et al. Effect of Volatile Organic Compounds Adsorption on 3D-Printed Pegda:Pedot for Long-Term Monitoring Devices. Nanomaterials 2021, 11, 94. [Google Scholar] [CrossRef]
- Battistoni, S.; Cocuzza, M.; Marasso, S.L.; Verna, A.; Erokhin, V. The Role of the Internal Capacitance in Organic Memristive Device for Neuromorphic and Sensing Applications. Adv. Electron. Mater. 2021, 7, 2100494. [Google Scholar] [CrossRef]
- Poddar, A.K.; Patel, S.S.; Patel, H.D. Synthesis, Characterization and Applications of Conductive Polymers: A Brief Review. Polym. Adv. Technol. 2021, 32, 4616–4641. [Google Scholar] [CrossRef]
- Kar, P. Doping in Conjugated Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Passeri, D.; Biagioni, A.; Rossi, M.; Tamburri, E.; Terranova, M.L. Characterization of Polyaniline-Detonation Nanodiamond Nanocomposite Fibers by Atomic Force Microscopy Based Techniques. Eur. Polym. J. 2013, 49, 991–998. [Google Scholar] [CrossRef]
- Passeri, D.; Tamburri, E.; Terranova, M.L.; Rossi, M. Polyaniline–Nanodiamond Fibers Resulting from the Self-Assembly of Nano-Fibrils: A Nanomechanical Study. Nanoscale 2015, 7, 14358–14367. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.C.D.; Ramaneti, R.; Baggerman, J.; Van Der Bent, J.F.; Marcelis, A.T.M.; Tong, H.D.; Van Rijn, C.J.M. Carbon Dioxide Sensing with Sulfonated Polyaniline. Sens. Actuators B Chem. 2012, 168, 123–130. [Google Scholar] [CrossRef]
- Yue, J.; Wang, Z.H.; Cromack, K.R.; Epstein, A.J.; MacDiarmid, A.G. Effect of Sulfonic Acid Group on Polyaniline Backbone. J. Am. Chem. Soc. 1991, 113, 2665–2671. [Google Scholar] [CrossRef]
- Wei, X.L.; Wang, Y.Z.; Long, S.M.; Bobeczko, C.; Epstein, A.J. Synthesis and Physical Properties of Highly Sulfonated Polyaniline. J. Am. Chem. Soc. 1996, 118, 2545–2555. [Google Scholar] [CrossRef]
- Wei, X.; Epstein, A.J. Synthesis of Highly Sulfonated Polyaniline. Synth. Met. 1995, 74, 123–125. [Google Scholar] [CrossRef]
- Yue, J.; Epstein, A.J. Synthesis of Self-Doped Conducting Polyaniline. J. Am. Chem. Soc. 1990, 112, 2800–2801. [Google Scholar] [CrossRef]
- Yang, Y.; Min, Y.; Wu, J.C.; Hansford, D.J.; Feinberg, S.E.; Epstein, A.J. Synthesis and Characterization of Cytocompatible Sulfonated Polyanilines. Macromol. Rapid Commun. 2011, 32, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Bernard, M.C.; Hugot-Le Goff, A. Quantitative Characterization of Polyaniline Films Using Raman Spectroscopy: II. Effects of Self-Doping in Sulfonated Polyaniline. Electrochim. Acta 2006, 52, 728–735. [Google Scholar] [CrossRef]
- Yue, J.; Gordon, G.; Epstein, A.J. Comparison of Different Synthetic Routes for Sulphonation of Polyaniline. Polymer 1992, 33, 4410–4418. [Google Scholar] [CrossRef]
- Brochocka, A.; Nowak, A.; Zajączkowska, H.; Sieradzka, M. Chemosensitive Thin Films Active to Ammonia Vapours. Sensors 2021, 21, 2948. [Google Scholar] [CrossRef] [PubMed]
- Medi, B.; Bahramian, A.; Nazari, V. Synthesis and Characterization of Conducting Polyaniline Nanostructured Thin Films for Solar Cell Applications. JOM 2021, 73, 504–514. [Google Scholar] [CrossRef]
- Akhlaq, M.; Khan, Z.S.; Mohamed, A.; Ibrahim, M.; Fouly, A.; Mohamed, A.; Fathelbab, A.M.R.; Akber, H.J.; Ibrahim, I.M.; Razeg, K.H. Hydrothermal Synthesis of Polyaniline Nano-Fibers as H2S Gas Sensor You May Also like Synthesis and Characterization of Electro-Spun TiO2 and TiO2-SnO2 Composite Nano-Fibers for Application in Advance Generation Solar Cells Enhancing the Tribological Performance of Epoxy Composites Utilizing Carbon Nano Fibers Additives for Journal Bearings Hydrothermal Synthesis of Polyaniline Nano-Fibers as H 2 S Gas Sensor. J. Phys. Conf. Ser. 2020, 1664, 12017. [Google Scholar] [CrossRef]
- Battistoni, S.; Verna, A.; Marasso, S.L.; Cocuzza, M.; Erokhin, V. On the Interpretation of Hysteresis Loop for Electronic and Ionic Currents in Organic Memristive Devices. Phys. Status Solidi 2020, 217, 1900985. [Google Scholar] [CrossRef]
- Battistoni, S.; Erokhin, V.; Iannotta, S. Emulation with Organic Memristive Devices of Impairment of LTP Mechanism in Neurodegenerative Disease Pathology. Neural Plast. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Kadri, Y.; Bekri-Abbess, I.; Herrasti, P. Highly Sensitive Enzyme-Free Sensor Based on a Carbon Paste Electrode Modified with Binary Zinc Oxide/Polyaniline Nanocomposites for Dopamine, Ascorbic Acid and Uric Acid Sensing. Electroanalysis 2022. [Google Scholar] [CrossRef]
- Chi, M.; Zhu, Y.; Jing, L.; Wang, C.; Lu, X. Fabrication of Oxidase-like Polyaniline-MnO2 Hybrid Nanowires and Their Sensitive Colorimetric Detection of Sulfite and Ascorbic Acid. Talanta 2019, 191, 171–179. [Google Scholar] [CrossRef]
- Aryal, K.P.; Jeong, H.K. Simultaneous Determination of Ascorbic Acid, Dopamine, and Uric Acid with Polyaniline/Hemin/Reduced Graphite Oxide Composite. Chem. Phys. Lett. 2021, 768, 138405. [Google Scholar] [CrossRef]
- Shen, Y.; Zheng, L. Polyaniline-Poly (Methylene Blue) Nano-Rod Composites as an Electrochemical Sensor for Sensitive Determination of Ascorbic Acid. Int. J. Electrochem. Sci. 2023, 18, 6–12. [Google Scholar] [CrossRef]
- Naghib, S.M.; Behzad, F.; Rahmanian, M.; Zare, Y.; Rhee, K.Y. A Highly Sensitive Biosensor Based on Methacrylated Graphene Oxide-Grafted Polyaniline for Ascorbic Acid Determination. Nanotechnol. Rev. 2020, 9, 760–767. [Google Scholar] [CrossRef]
- Bilal, S.; Akbar, A.; Shah, A.U.H.A. Highly Selective and Reproducible Electrochemical Sensing of Ascorbic Acid Through a Conductive Polymer Coated Electrode. Polymers 2019, 11, 1346. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Nadiger, V.G.; Goswami, D.; Martinez, R.V. Conformal, Waterproof Electronic Decals for Wireless Monitoring of Sweat and Vaginal PH at the Point-of-Care. Biosens. Bioelectron. 2020, 160, 112206. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Nyein, H.Y.Y.; Shahpar, Z.; Tai, L.C.; Wu, E.; Bariya, M.; Ota, H.; Fahad, H.M.; Chen, K.; Javey, A. Wearable Sweat Biosensors. Tech. Dig. Int. Electron Devices Meet. IEDM 2017, 6.6.1–6.6.4. [Google Scholar] [CrossRef]
- Roman, S.; Gyawali, C.P.; Savarino, E.; Yadlapati, R.; Zerbib, F.; Wu, J.; Vela, M.; Tutuian, R.; Tatum, R.; Sifrim, D.; et al. Ambulatory Reflux Monitoring for Diagnosis of Gastro-Esophageal Reflux Disease: Update of the Porto Consensus and Recommendations from an International Consensus Group. Neurogastroenterol. Motil. 2017, 29, 1–15. [Google Scholar] [CrossRef]
- Rose, C.; Parker, A.; Jefferson, B.; Cartmell, E. The Characterization of Feces and Urine: A Review of the Literature to Inform Advanced Treatment Technology. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1827–1879. [Google Scholar] [CrossRef]
- Qiu, S.; Chen, C.; Zheng, W.; Li, W.; Zhao, H.; Wang, L. Long-Term Corrosion Protection of Mild Steel by Epoxy Coating Containing Self-Doped Polyaniline Nanofiber. Synth. Met. 2017, 229, 39–46. [Google Scholar] [CrossRef]
- Liao, Y.; Strong, V.; Chian, W.; Wang, X.; Li, X.G.; Kaner, R.B. Sulfonated Polyaniline Nanostructures Synthesized via Rapid Initiated Copolymerization with Controllable Morphology, Size, and Electrical Properties. Macromolecules 2012, 45, 1570–1579. [Google Scholar] [CrossRef]
- Lindfors, T.; Ivaska, A. Raman Based PH Measurements with Polyaniline. J. Electroanal. Chem. 2005, 580, 320–329. [Google Scholar] [CrossRef]
- Tamburri, E.; Orlanducci, S.; Guglielmotti, V.; Reina, G.; Rossi, M.; Terranova, M.L. Engineering Detonation Nanodiamond—Polyaniline Composites by Electrochemical Routes: Structural Features and Functional Characterizations. Polymer. 2011, 52, 5001–5008. [Google Scholar] [CrossRef]
- Tamburri, E.; Guglielmotti, V.; Orlanducci, S.; Terranova, M.L.; Sordi, D.; Passeri, D.; Matassa, R.; Rossi, M. Nanodiamond-Mediated Crystallization in Fibers of PANI Nanocomposites Produced by Template-Free Polymerization: Conductive and Thermal Properties of the Fibrillar Networks. Polymer 2012, 53, 4045–4053. [Google Scholar] [CrossRef]
- Józefowicz, M.E.; Epstein, A.J.; Pouget, J.P.; Masters, J.G.; Ray, A.; Sun, Y.; Tang, X.; Macdiarmid, A.G. X-ray Structure of Polyanilines. Synth. Met. 1991, 41, 723–726. [Google Scholar] [CrossRef]
- Freund, M.S.; Deore, F. Self-Doped Conductive Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2007; p. 315. [Google Scholar]
- Heffner, G.W.; Dahman, S.J.; Pearson, D.S.; Gettinger, C.L. The Effect of Molecular Weight and Crystallinity on the Conductivity of a Conducting Polymer. Polymer 1993, 34, 3155–3159. [Google Scholar] [CrossRef]
- Fan, F.; Wang, W.; Holt, A.P.; Feng, H.; Uhrig, D.; Lu, X.; Hong, T.; Wang, Y.; Kang, N.G.; Mays, J.; et al. Effect of Molecular Weight on the Ion Transport Mechanism in Polymerized Ionic Liquids. Macromolecules 2016, 49, 4557–4570. [Google Scholar] [CrossRef]
- Du, X.; Xu, Y.; Xiong, L.; Bai, Y.; Zhu, J.; Mao, S. Polyaniline with High Crystallinity Degree: Synthesis, Structure, and Electrochemical Properties. J. Appl. Polym. Sci 2014, 131, 40827. [Google Scholar] [CrossRef]
- Popov, A.; Brasiunas, B.; Mikoliunaite, L.; Bagdziunas, G.; Ramanavicius, A.; Ramanaviciene, A. Comparative Study of Polyaniline (PANI), Poly(3,4-Ethylenedioxythiophene) (PEDOT) and PANI-PEDOT Films Electrochemically Deposited on Transparent Indium Thin Oxide Based Electrodes. Polymer 2019, 172, 133–141. [Google Scholar] [CrossRef]
- Chen, W.C.; Wen, T.C.; Hu, C.C.; Gopalan, A. Identification of Inductive Behavior for Polyaniline via Electrochemical Impedance Spectroscopy. Electrochim. Acta 2002, 47, 1305–1315. [Google Scholar] [CrossRef]
- Kuzmany, H.; Sariciftci, N.S. In Situ Spectro-Electrochemical Studies of Polyaniline. Synth. Met. 1987, 18, 353–358. [Google Scholar] [CrossRef]
- Biabangard, F.; Nazari, H.; Arefinia, R. Effect of PH on the Electrochemical Properties of Polyaniline Nanoparticle Suspension in Strongly Acidic Solution: An Experimental and Theoretical Study. J. Solid State Electrochem. 2021, 25, 881–893. [Google Scholar] [CrossRef]
- Yan, B.; Yang, J.; Li, Y.; Cao, Y. Electrochemical Adsorption of Hydrogen and Various Ions on Polyaniline Film. Reactions Concerning the First Pair of Cyclic Voltammetric Peaks. Synth. Met. 1991, 44, 189–197. [Google Scholar] [CrossRef]
- Jamadade, V.S.; Dhawale, D.S.; Lokhande, C.D. Studies on Electrosynthesized Leucoemeraldine, Emeraldine and Pernigraniline Forms of Polyaniline Films and Their Supercapacitive Behavior. Synth. Met. 2010, 160, 955–960. [Google Scholar] [CrossRef]
- Li, C.; Mu, S. The Electrochemical Activity of Sulfonic Acid Ring-Substituted Polyaniline in the Wide PH Range. Synth. Met. 2005, 149, 143–149. [Google Scholar] [CrossRef]
- Xue, P.; Zhang, X.; Chuah, Y.J.; Wu, Y.; Kang, Y. Flexible PEGDA-Based Microneedle Patches with Detachable PVP–CD Arrowheads for Transdermal Drug Delivery. RSC Adv. 2015, 5, 75204–75209. [Google Scholar] [CrossRef]
- Pescosolido, F.; Montaina, L.; Carcione, R.; Politi, S.; Matassa, R.; Carotenuto, F.; Nottola, S.A.; Di Nardo, P.; Tamburri, E.; Pescosolido, F.; et al. A New Strong-Acid Free Route to Produce Xanthan Gum-PANI Composite Scaffold Supporting Bioelectricity. Macromol. Biosci. 2023, 7, 2300132. [Google Scholar] [CrossRef]
- Mir, A.; Kumar, A.; Riaz, U. A Short Review on the Synthesis and Advance Applications of Polyaniline Hydrogels. RSC Adv. 2022, 12, 19122–19132. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, Y.; Guo, Q.; Cai, X.; Xiao, J.; Ding, Z.; Yang, J. Macroporous Double-Network Hydrogel for High-Efficiency Solar Steam Generation under 1 Sun Illumination. ACS Appl. Mater. Interfaces 2018, 10, 10998–11007. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, S.; Hong, R. Graphene Oxide/Polyaniline Nanocomposites Used in Anticorrosive Coatings for Environmental Protection. Coatings 2020, 10, 1215. [Google Scholar] [CrossRef]
- Pyarasani, R.D.; Jayaramudu, T.; John, A. Polyaniline-Based Conducting Hydrogels. J. Mater. Sci. 2018, 54, 974–996. [Google Scholar] [CrossRef]
- Cochrane, C.; Koncar, V.; Lewandowski, M.; Dufour, C. Design and Development of a Flexible Strain Sensor for Textile Structures Based on a Conductive Polymer Composite. Sensors 2007, 7, 473–492. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carcione, R.; Pescosolido, F.; Montaina, L.; Toschi, F.; Orlanducci, S.; Tamburri, E.; Battistoni, S. Self-Standing 3D-Printed PEGDA–PANIs Electroconductive Hydrogel Composites for pH Monitoring. Gels 2023, 9, 784. https://doi.org/10.3390/gels9100784
Carcione R, Pescosolido F, Montaina L, Toschi F, Orlanducci S, Tamburri E, Battistoni S. Self-Standing 3D-Printed PEGDA–PANIs Electroconductive Hydrogel Composites for pH Monitoring. Gels. 2023; 9(10):784. https://doi.org/10.3390/gels9100784
Chicago/Turabian StyleCarcione, Rocco, Francesca Pescosolido, Luca Montaina, Francesco Toschi, Silvia Orlanducci, Emanuela Tamburri, and Silvia Battistoni. 2023. "Self-Standing 3D-Printed PEGDA–PANIs Electroconductive Hydrogel Composites for pH Monitoring" Gels 9, no. 10: 784. https://doi.org/10.3390/gels9100784