Enhanced Osteogenesis Potential of MG-63 Cells through Sustained Delivery of VEGF via Liposomal Hydrogel
Abstract
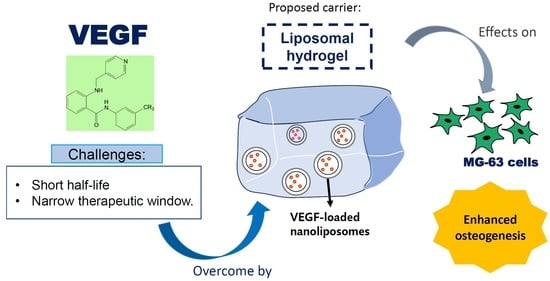
:1. Introduction
2. Results
2.1. Characterization of Scaffold
2.1.1. Characterization of VEGF-Loaded Liposomes
2.1.2. Microstructure and Porosity of Hydrogels
2.1.3. Chemical Properties of Hydrogels Using FTIR Spectroscopy
2.1.4. Release Profile of VEGF from the Hydrogels
2.2. In-Vitro Characterization of Cell–Hydrogel 2.5D Culture
2.2.1. Effect of Sustained Delivery of VEGF on Cell Morphology
2.2.2. Effect of Sustained Delivery of VEGF on Cell Viability
2.2.3. Effect of Sustained Delivery of VEGF on ALP Activity
2.2.4. Effect of Sustained Delivery of VEGF on Matrix Mineralization
3. Discussions
3.1. Characterization of Scaffold
3.2. Effects of Liposomal Hydrogel on Cell Viability, Differentiation, and Mineralization
3.3. Limitations and Recommendations for Future Research
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Preparation of Scaffold
5.2.1. Preparation of VEGF-Loaded Liposomes
5.2.2. Preparation of Thermoresponsive Hydrogels
5.3. Characterization of Scaffold
5.3.1. Characterization of Physical Properties and Morphology of Liposomes
5.3.2. Morphological Characterization of Hydrogel Using FESEM
5.3.3. Porosity Determination of Hydrogels
5.3.4. Characterization of Chemical Properties of Hydrogels Using FTIR Spectroscopy
5.3.5. Release Profile of VEGF from the Hydrogels
5.4. Cell–Hydrogel 2.5D Co-Culture
5.5. In Vitro Characterization of Cell–Hydrogel Co-Culture
5.5.1. Cell Morphology Analysis Using FESEM
5.5.2. Cell Viability Analysis Using MTT Assay
5.5.3. Cell Differentiation Analysis Using Alkaline Phosphatase (ALP) Assay
5.5.4. Mineralization Analysis Using Alizarin Red S Staining Quantification
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pacelli, S.; Basu, S.; Whitlow, J.; Chakravarti, A.; Acosta, F.; Varshney, A.; Modaresi, S.; Berkland, C.; Paul, A. Strategies to develop endogenous stem cell-recruiting bioactive materials for tissue repair and regeneration. Adv. Drug Deliv. Rev. 2017, 120, 50–70. [Google Scholar] [CrossRef] [PubMed]
- Luque-Martín, E.; Tobella-Camps, M.-L.; Rivera-Baró, A. Alveolar graft in the cleft lip and palate patient: Review of 104 cases. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e531–e537. [Google Scholar] [CrossRef] [PubMed]
- Wahab, R.M.A.; Ng, W.; Yazid, F.; Luchman, N.; Ariffin, S. Orthodontic considerations in bone graft selection for alveolar cleft repair. Sains Malays. 2020, 49, 349–356. [Google Scholar] [CrossRef]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef]
- Myeroff, C.; Archdeacon, M. Autogenous bone graft: Donor sites and techniques. J. Bone Jt. Surg. Am. Vol. 2011, 93, 2227–2236. [Google Scholar] [CrossRef]
- Mankin, H.J.; Hornicek, F.J.; Raskin, K.A. Infection in massive bone allografts. Clin. Orthop. Relat. Res. 2005, 432, 210–216. [Google Scholar] [CrossRef]
- Wahab, R.M.A.; Abdullah, N.; Zainal Ariffin, S.H.; Che Abdullah, C.A.; Yazid, F. Effects of the sintering process on nacre-derived hydroxyapatite scaffolds for bone engineering. Molecules 2020, 25, 3129. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef]
- Oppenheimer, A.J.; Mesa, J.; Buchman, S.R. Current and emerging basic science concepts in bone biology: Implications in craniofacial surgery. J. Craniofacial Surg. 2012, 23, 30–36. [Google Scholar] [CrossRef]
- Stamnitz, S.; Klimczak, A. Mesenchymal Stem Cells, Bioactive Factors, and Scaffolds in Bone Repair: From Research Perspectives to Clinical Practice. Cells 2021, 10, 1925. [Google Scholar] [CrossRef]
- Gromolak, S.; Krawczenko, A.; Antończyk, A.; Buczak, K.; Kiełbowicz, Z.; Klimczak, A. Biological Characteristics and Osteogenic Differentiation of Ovine Bone Marrow Derived Mesenchymal Stem Cells Stimulated with FGF-2 and BMP-2. Int. J. Mol. Sci. 2020, 21, 9726. [Google Scholar] [CrossRef] [PubMed]
- Schipani, E.; Maes, C.; Carmeliet, G.; Semenza, G.L. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J. Bone Miner. Res. 2009, 24, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Grosso, A.; Burger, M.G.; Lunger, A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. It takes two to tango: Coupling of angiogenesis and osteogenesis for bone regeneration. Front. Bioeng. Biotechnol. 2017, 5, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldo, B.A. Side effects of cytokines approved for therapy. Drug Saf. 2014, 37, 921–943. [Google Scholar] [CrossRef]
- Hassani, A.; Avci Ç, B.; Kerdar, S.N.; Amini, H.; Amini, M.; Ahmadi, M.; Sakai, S.; Bagca, B.G.; Ozates, N.P.; Rahbarghazi, R.; et al. Interaction of alginate with nano-hydroxyapatite-collagen using strontium provides suitable osteogenic platform. J. Nanobiotechnol. 2022, 20, 310. [Google Scholar] [CrossRef]
- Oliveira, M.Z.; Albano, M.B.; Namba, M.M.; da Cunha, L.A.; de Lima Gonçalves, R.R.; Trindade, E.S.; Andrade, L.F.; Vidigal, L. Effect of hyaluronic acids as chondroprotective in experimental model of osteoarthrosis. Rev. Bras. Ortop. 2014, 49, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Vieira, S.; Strymecka, P.; Stanaszek, L.; Silva-Correia, J.; Drela, K.; Fiedorowicz, M.; Malysz-Cymborska, I.; Janowski, M.; Reis, R.L.; Łukomska, B.; et al. Mn-Based Methacrylated Gellan Gum Hydrogels for MRI-Guided Cell Delivery and Imaging. Bioengineering 2023, 10, 427. [Google Scholar] [CrossRef]
- Alavi, S.; Haeri, A.; Dadashzadeh, S. Utilization of chitosan-caged liposomes to push the boundaries of therapeutic delivery. Carbohydr. Polym. 2017, 157, 991–1012. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and chitosan: Production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Saravanan, S.; Vimalraj, S.; Thanikaivelan, P.; Banudevi, S.; Manivasagam, G. A review on injectable chitosan/beta glycerophosphate hydrogels for bone tissue regeneration. Int. J. Biol. Macromol. 2019, 121, 38–54. [Google Scholar] [CrossRef]
- Thirumaleshwar, S.; Kulkarni, P.; Gowda, D. Liposomal hydrogels: A novel drug delivery system for wound dressing. Curr. Drug Ther. 2012, 7, 212–218. [Google Scholar] [CrossRef]
- Robert, A.W.; Angulski, A.B.B.; Spangenberg, L.; Shigunov, P.; Pereira, I.T.; Bettes, P.S.L.; Naya, H.; Correa, A.; Dallagiovanna, B.; Stimamiglio, M.A. Gene expression analysis of human adipose tissue-derived stem cells during the initial steps of in vitro osteogenesis. Sci. Rep. 2018, 8, 4739. [Google Scholar] [CrossRef]
- Ruel-Gariépy, E.; Chenite, A.; Chaput, C.; Guirguis, S.; Leroux, J. Characterization of thermosensitive chitosan gels for the sustained delivery of drugs. Int. J. Pharm. 2000, 203, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Nakhaei, P.; Margiana, R.; Bokov, D.O.; Abdelbasset, W.K.; Jadidi Kouhbanani, M.A.; Varma, R.S.; Marofi, F.; Jarahian, M.; Beheshtkhoo, N. Liposomes: Structure, Biomedical Applications, and Stability Parameters with Emphasis on Cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 705886. [Google Scholar] [CrossRef]
- Marasini, N.; Giddam, A.K.; Ghaffar, K.A.; Batzloff, M.R.; Good, M.F.; Skwarczynski, M.; Toth, I. Multilayer engineered nanoliposomes as a novel tool for oral delivery of lipopeptide-based vaccines against group A Streptococcus. Nanomedicine 2016, 11, 1223–1236. [Google Scholar] [CrossRef]
- Briuglia, M.L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Bahari, L.A.S.; Hamishehkar, H. The impact of variables on particle size of solid lipid nanoparticles and nanostructured lipid carriers; A comparative literature review. Adv. Pharm. Bull. 2016, 6, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems—A review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Tan, Y.; Xu, K.; Lu, C.; Wang, P. In situ hydrogel constructed by starch-based nanoparticles via a Schiff base reaction. Carbohydr. Polym. 2014, 110, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Iviglia, G.; Kargozar, S.; Baino, F. Biomaterials, current strategies, and novel nano-technological approaches for periodontal regeneration. J. Funct. Biomater. 2019, 10, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, A.; Melo, J.S. Preparation of a sponge-like biocomposite agarose–chitosan scaffold with primary hepatocytes for establishing an in vitro 3D liver tissue model. RSC Adv. 2015, 5, 30701–30710. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Kowalczuk, D.; Pitucha, M. Application of FTIR method for the assessment of immobilization of active substances in the matrix of biomedical materials. Materials 2019, 12, 2972. [Google Scholar] [CrossRef] [Green Version]
- Qin, H.; Wang, J.; Wang, T.; Gao, X.; Wan, Q.; Pei, X. Preparation and characterization of chitosan/β-glycerophosphate thermal-sensitive hydrogel reinforced by graphene oxide. Front. Chem. 2018, 6, 565. [Google Scholar] [CrossRef] [Green Version]
- Pilch, E.; Musiał, W. Selected Physicochemical Properties of Lyophilized Hydrogel with Liposomal Fraction of Calcium Dobesilate. Materials 2018, 11, 2143. [Google Scholar] [CrossRef] [Green Version]
- Boido, M.; Ghibaudi, M.; Gentile, P.; Favaro, E.; Fusaro, R.; Tonda-Turo, C. Chitosan-based hydrogel to support the paracrine activity of mesenchymal stem cells in spinal cord injury treatment. Sci. Rep. 2019, 9, 6402. [Google Scholar] [CrossRef] [Green Version]
- Assaad, E.; Maire, M.; Lerouge, S. Injectable thermosensitive chitosan hydrogels with controlled gelation kinetics and enhanced mechanical resistance. Carbohydr. Polym. 2015, 130, 87–96. [Google Scholar] [CrossRef]
- Cheng, R.; Yan, Y.; Liu, H.; Chen, H.; Pan, G.; Deng, L.; Cui, W. Mechanically enhanced lipo-hydrogel with controlled release of multi-type drugs for bone regeneration. Appl. Mater. Today 2018, 12, 294–308. [Google Scholar] [CrossRef]
- Melling, G.E.; Colombo, J.S.; Avery, S.J.; Ayre, W.N.; Evans, S.L.; Waddington, R.J.; Sloan, A.J. Liposomal delivery of demineralized dentin matrix for dental tissue regeneration. Tissue Eng. Part A 2018, 24, 1057–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Tsao, H.; Kang, Y.; Young, D.A.; Sen, M.; Wenke, J.C.; Yang, Y. In vitro evaluation of an injectable chitosan gel for sustained local delivery of BMP-2 for osteoblastic differentiation. J. Biomed. Mater. Res. Part A 2011, 99B, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Zhou, Y.; Yu, Y.; Zhou, X.; Du, W.; Wan, M.; Fan, Y.; Zhou, X.; Xu, X.; Zheng, L. Evaluation of chitosan hydrogel for sustained delivery of VEGF for odontogenic differentiation of dental pulp stem cells. Stem Cells Int. 2019, 2019, 1515040. [Google Scholar] [CrossRef] [Green Version]
- Elango, J. Proliferative and Osteogenic Supportive Effect of VEGF-Loaded Collagen-Chitosan Hydrogel System in Bone Marrow Derived Mesenchymal Stem Cells. Pharmaceutics 2023, 15, 1297. [Google Scholar] [CrossRef]
- D’ Alimonte, I.; Nargi, E.; Mastrangelo, F.; Falco, G.; Lanuti, P.; Marchisio, M.; Miscia, S.; Robuffo, I.; Capogreco, M.; Buccella, S.; et al. Vascular endothelial growth factor enhances in vitro proliferation and osteogenic differentiation of human dental pulp stem cells. J. Biol. Regul. Homeost. Agents 2011, 25, 57–69. [Google Scholar]
- Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, Á. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Loh, E.Y.X.; Mohamad, N.; Fauzi, M.B.; Ng, M.H.; Ng, S.F.; Mohd Amin, M.C.I. Development of a bacterial cellulose-based hydrogel cell carrier containing keratinocytes and fibroblasts for full-thickness wound healing. Sci. Rep. 2018, 8, 2875. [Google Scholar] [CrossRef] [Green Version]
- Kozhukharova, I.; Minkevich, N.; Alekseenko, L.; Domnina, A.; Lyublinskaya, O. Paracrine and autocrine effects of VEGF are enhanced in human eMSC spheroids. Int. J. Mol. Sci. 2022, 23, 14324. [Google Scholar] [CrossRef]
- Jacobsen, K.A.; Al-Aql, Z.S.; Wan, C.; Fitch, J.L.; Stapleton, S.N.; Mason, Z.D.; Cole, R.M.; Gilbert, S.R.; Clemens, T.L.; Morgan, E.F.; et al. Bone formation during distraction osteogenesis is dependent on both VEGFR1 and VEGFR2 signaling. J. Bone Miner. Res. 2008, 23, 596–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persson, M.; Lehenkari, P.P.; Berglin, L.; Turunen, S.; Finnilä, M.A.J.; Risteli, J.; Skrifvars, M.; Tuukkanen, J. Osteogenic differentiation of human mesenchymal stem cells in a 3D woven scaffold. Sci. Rep. 2018, 8, 10457. [Google Scholar] [CrossRef] [Green Version]
- Hanna, H.; Mir, L.M.; Andre, F.M. In vitro osteoblastic differentiation of mesenchymal stem cells generates cell layers with distinct properties. Stem Cell Res. Ther. 2018, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Takagishi, Y.; Kawakami, T.; Hara, Y.; Shinkai, M.; Takezawa, T.; Nagamune, T. Bone-like tissue formation by three-dimensional culture of MG63 osteosarcoma cells in gelatin hydrogels using calcium-enriched medium. Tissue Eng. 2006, 12, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Orimo, H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J. Nippon. Med. Sch. 2010, 77, 4–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.L.; Stanford, C.M.; Keller, J.C. Calcium and phosphate supplementation promotes bone cell mineralization: Implications for hydroxyapatite (HA)-enhanced bone formation. J. Biomed. Mater. Res. 2000, 52, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Aksel, H.; Huang, G.T. Combined effects of vascular endothelial growth factor and bone morphogenetic protein 2 on odonto/osteogenic differentiation of human dental pulp stem cells in vitro. J. Endod. 2017, 43, 930–935. [Google Scholar] [CrossRef]
- Song, X.; Liu, S.; Qu, X.; Hu, Y.; Zhang, X.; Wang, T.; Wei, F. BMP2 and VEGF promote angiogenesis but retard terminal differentiation of osteoblasts in bone regeneration by up-regulating Id1. Acta Biochim. Et Biophys. Sin. 2011, 43, 796–804. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Um, S.; Jang, J.; Seo, B.M. Effects of VEGF and FGF-2 on proliferation and differentiation of human periodontal ligament stem cells. Cell Tissue Res. 2012, 348, 475–484. [Google Scholar] [CrossRef]
- Behr, B.; Tang, C.; Germann, G.; Longaker, M.T.; Quarto, N. Locally applied vascular endothelial growth factor A increases the osteogenic healing capacity of human adipose-derived stem cells by promoting osteogenic and endothelial differentiation. Stem Cells 2011, 29, 286–296. [Google Scholar] [CrossRef] [Green Version]
- Dreyer, C.H.; Kjaergaard, K.; Ding, M.; Qin, L. Vascular endothelial growth factor for in vivo bone formation: A systematic review. J. Orthop. Transl. 2020, 24, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Goossens, S.; Bartunkova, S.; Drogat, B.; Coenegrachts, L.; Stockmans, I.; Moermans, K.; Nyabi, O.; Haigh, K.; Naessens, M.; et al. Increased skeletal VEGF enhances beta-catenin activity and results in excessively ossified bones. EMBO J. 2010, 29, 424–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šturm, L.; Poklar Ulrih, N. Basic methods for preparation of liposomes and studying their interactions with different compounds, with the emphasis on polyphenols. Int. J. Mol. Sci. 2021, 22, 6547. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.; Ma, Y.; Bruekers, S.M.C.; Ma, S.; Huck, W.T.S. 25th Anniversary Article: Designer Hydrogels for Cell Cultures: A Materials Selection Guide. Adv. Mater. 2014, 26, 125–148. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.T.; Hutmacher, D.W. A comparison of micro CT with other techniques used in the characterization of scaffolds. Biomaterials 2006, 27, 1362–1376. [Google Scholar] [CrossRef]
- Hadzir, S.N.; Ibrahim, S.N.; Abdul Wahab, R.M.; Zainol Abidin, I.Z.; Senafi, S.; Ariffin, Z.Z.; Abdul Razak, M.; Zainal Ariffin, S.H. Ascorbic acid induces osteoblast differentiation of human suspension mononuclear cells. Cytotherapy 2014, 16, 674–682. [Google Scholar] [CrossRef]








| Formulation | Particle Sizes (d·nm) | PDI | Zeta-Potential (mV) | p-Value |
|---|---|---|---|---|
| Liposomes without VEGF | 114 ± 28 | 0.221 ± 0.01 | 30.7 ± 12.8 | p > 0.05 |
| Liposomes with VEGF | 171 ± 31 | 0.174 ± 0.03 | 38.5 ± 12.9 |
| Hydrogel Formulation | Pore Size (µm) | Porosity (%) | p-Value |
|---|---|---|---|
| Hydrogel without liposomes + VEGF | 201.4 ± 24.5 | 81.1 ± 3.3 | p > 0.05 |
| Liposomal hydrogel + VEGF | 205.8 ± 25.5 | 84.0 ± 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, M.H.; Megat Abdul Wahab, R.; Zainal Ariffin, S.H.; Azmi, F.; Yazid, F. Enhanced Osteogenesis Potential of MG-63 Cells through Sustained Delivery of VEGF via Liposomal Hydrogel. Gels 2023, 9, 562. https://doi.org/10.3390/gels9070562
Tsai MH, Megat Abdul Wahab R, Zainal Ariffin SH, Azmi F, Yazid F. Enhanced Osteogenesis Potential of MG-63 Cells through Sustained Delivery of VEGF via Liposomal Hydrogel. Gels. 2023; 9(7):562. https://doi.org/10.3390/gels9070562
Chicago/Turabian StyleTsai, Milton Hongli, Rohaya Megat Abdul Wahab, Shahrul Hisham Zainal Ariffin, Fazren Azmi, and Farinawati Yazid. 2023. "Enhanced Osteogenesis Potential of MG-63 Cells through Sustained Delivery of VEGF via Liposomal Hydrogel" Gels 9, no. 7: 562. https://doi.org/10.3390/gels9070562