Evaluation of Physicochemical Properties of a Hydrocolloid-Based Functional Food Fortified with Caulerpa lentillifera: A D-Optimal Design Approach
Abstract
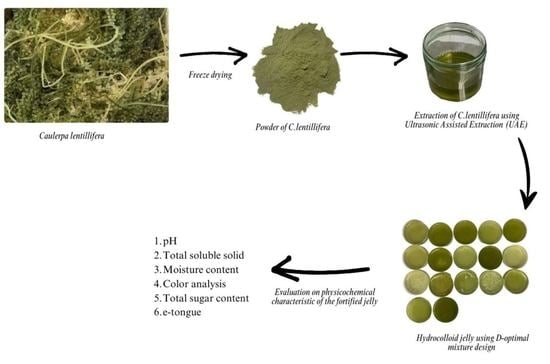
:1. Introduction
2. Results and Discussion
2.1. Analysis of the Adequacy of the Fitted Model
2.2. Agreement between Model Prediction and Observed Value
2.3. D-Optimal Analysis
2.3.1. pH Response Analysis
2.3.2. Total Soluble Solid (TSS) Response Analysis
2.3.3. Moisture Analysis
2.4. Selection of the Design
2.5. Verification of Constructed Model
2.6. Color Analysis
2.7. Sugar Analysis Using HPLC
2.8. E-Tongue
3. Conclusions
4. Materials and Methods
4.1. Experimental Materials
4.2. Extraction of C. lentillifera
4.3. Selection of Excipients
4.4. Preparation of Jellies Fortified with C. lentillifera
4.5. Physicochemical Analysis
4.6. Sugar Analysis
4.7. Electric Tongue Sensor
4.8. Experimental Design and Statistical Analysis
4.9. Verification of the Model
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Syakilla, N.; George, R.; Chye, F.Y.; Pindi, W.; Mantihal, S.; Wahab, N.A.; Fadzwi, F.M.; Gu, P.H.; Matanjun, P. A Review on Nutrients, Phytochemicals, and Health Benefits of Green Seaweed, Caulerpa lentillifera. Foods 2022, 11, 2832. [Google Scholar] [CrossRef]
- Biancacci, C.; Visch, W.; Callahan, D.L.; Farrington, G.; Francis, D.S.; Lamb, P.; McVilly, A.; Nardelli, A.; Sanderson, J.C.; Schwoerbel, J.; et al. Optimisation of At-Sea Culture and Harvest Conditions for Cultivated Macrocystis Pyrifera: Yield, Biofouling and Biochemical Composition of Cultured Biomass. Front. Mar. Sci. 2022, 9, 951538. [Google Scholar] [CrossRef]
- Mabeau, S.; Fleurence, J. Seaweed in Food Products: Biochemical and Nutritional Aspects. Trends Food Sci. Technol. 1993, 4, 103–107. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Lee, W.W.; Jeon, Y.J. Nutrients and Bioactive Potentials of Edible Green and Red Seaweed in Korea. Fish. Aquat. Sci. 2018, 21, 19. [Google Scholar] [CrossRef]
- du Preez, R.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Caulerpa lentillifera (Sea Grapes) Improves Cardiovascular and Metabolic Health of Rats with Diet-Induced Metabolic Syndrome. Metabolites 2020, 10, 500. [Google Scholar] [CrossRef]
- Yap, W.F.; Tay, V.; Tan, S.H.; Yow, Y.Y.; Chew, J. Decoding Antioxidant and Antibacterial Potentials of Malaysian Green Seaweeds: Caulerpa racemosa and Caulerpa lentillifera. Antibiotics 2019, 8, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, H.; Gu, X.; Zhu, Z.; Wang, C.; Xia, X.; Zhou, N.; Liu, X.; Zhao, M. Effects of Bottom Sediment on the Accumulation of Nutrients in the Edible Green Seaweed Caulerpa lentillifera (Sea Grapes). J. Appl. Phycol. 2020, 32, 705–716. [Google Scholar] [CrossRef]
- de Gaillande, C.; Payri, C.; Remoissenet, G.; Zubia, M. Caulerpa Consumption, Nutritional Value and Farming in the Indo-Pacific Region. J. Appl. Phycol. 2017, 29, 2249–2266. [Google Scholar] [CrossRef]
- Stuthmann, L.E.; Springer, K.; Kunzmann, A. Cultured and Packed Sea Grapes (Caulerpa lentillifera): Effect of Different Irradiances on Photosynthesis. J. Appl. Phycol. 2021, 33, 1125–1136. [Google Scholar] [CrossRef]
- Tanduyan, S.N.; Gonzaga, R.B.; Bensig, V.D. Off Bottom Culture of Caulerpa lentillifera in Three Different Water Levels in the Marine Waters of San Francisco, Cebu, Philippines. Galaxea J. Coral Reef Stud. 2013, 15, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Liang, Z.; Liu, F.; Wang, W.; Zhang, P.; Yuan, Y.; Yao, H.; Sun, X.; Wang, F. A Reasonable Strategy for Caulerpa lentillifera J. Agardh (Bryopsidales, Chlorophyta) Transportation Based on the Biochemical and Photophysiological Responses to Dehydration Stress. Algal Res. 2021, 56, 102304. [Google Scholar] [CrossRef]
- Shinwari, K.J.; Rao, P.S. Stability of Bioactive Compounds in Fruit Jam and Jelly during Processing and Storage: A Review. Trends Food Sci. Technol. 2018, 75, 181–193. [Google Scholar] [CrossRef]
- Crary, M.; Sura, L.; Madhavan, A.; Carnaby-Mann, G. Dysphagia in the Elderly: Management and Nutritional Considerations. Clin. Interv. Flanging 2012, 7, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Fujioka, S.; Kadota, K.; Yoshida, M.; Shirakawa, Y. Improvement in the Elution Behavior of Rutin via Binary Amorphous Solid with Flavonoid Using a Mechanochemical Process. Food Bioprod. Process. 2020, 123, 274–283. [Google Scholar] [CrossRef]
- Miranda, J.S.; Costa, B.V.; de Oliveira, I.V.; de Lima, D.C.N.; Martins, E.M.F.; de Castro Leite Júnior, B.R.; do Nascimento Benevenuto, W.C.A.; de Queiroz, I.C.; da Silva, R.R.; Martins, M.L. Probiotic Jelly Candies Enriched with Native Atlantic Forest Fruits and Bacillus Coagulans GBI-30 6086. Lwt 2020, 126, 109275. [Google Scholar] [CrossRef]
- Shahsavani Mojarrad, L.; Rafe, A. Rheological Characteristics of Binary Composite Gels of Wheat Flour and High Amylose Corn Starch. J. Texture Stud. 2018, 49, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Nogami, S.; Uchiyama, H.; Kadota, K.; Tozuka, Y. Design of a PH-Responsive Oral Gel Formulation Based on the Matrix Systems of Gelatin/Hydroxypropyl Methylcellulose Phthalate for Controlled Drug Release. Int. J. Pharm. 2021, 592, 120047. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.A.; Bhat, R. Gelatin Alternatives for the Food Industry: Recent Developments, Challenges and Prospects. Trends Food Sci. Technol. 2008, 19, 644–656. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, D.; Guo, Q.; Liu, C. Textural and Structural Properties of a κ-carrageenan–Konjac Gum Mixed Gel: Effects of κ-carrageenan Concentration, Mixing Ratio, Sucrose and Ca2+ Concentrations and Its Application in Milk Pudding. J. Sci. Food Agric. 2021, 101, 3021–3029. [Google Scholar] [CrossRef]
- Borhan, F.P.; Abd Gani, S.S.; Shamsuddin, R. The Use of D-Optimal Mixture Design in Optimising Okara Soap Formulation for Stratum Corneum Application. Sci. World J. 2014, 2014, 173979. [Google Scholar] [CrossRef] [Green Version]
- Jesus, R.R.-A.; Ariadna, L.P.; Julio, C. sar E. A.; Humberto, M.Q.; Antonio, I.C.; Jose, C.T.C. Optimization of a Novel Tablets Formulation Using D-Optimal Mixture Design. Afr. J. Pharm. Pharmacol. 2015, 9, 474–483. [Google Scholar] [CrossRef]
- Zakaria, F.; Tan, J.K.; Mohd Faudzi, S.M.; Abdul Rahman, M.B.; Ashari, S.E. Ultrasound-Assisted Extraction Conditions Optimisation Using Response Surface Methodology from Mitragyna Speciosa (Korth.) Havil Leaves. Ultrason. Sonochem. 2021, 81, 105851. [Google Scholar] [CrossRef]
- Sriharti; Andriansyah, R.C.E.; Agustina, W.; Indriati, A.; Litaay, C.; Luthfiyanti, R.; Mayasti, N.K.I.; Triyono, A.; Tribowo, R.I.; Purwandoko, P.B. Optimization of Herbal Tea Drink Formula Based on Aloe Vera Rind (Aloe barbadensis miller). Food Sci. Technol. 2022, 42, e69022. [Google Scholar] [CrossRef]
- Kamairudin, N.; Gani, S.; Masoumi, H.; Hashim, P. Optimization of Natural Lipstick Formulation Based on Pitaya (Hylocereus polyrhizus) Seed Oil Using D-Optimal Mixture Experimental Design. Molecules 2014, 19, 16672–16683. [Google Scholar] [CrossRef] [Green Version]
- Rasidek, N.A.M.; Nordin, M.F.M.; Shameli, K. Formulation and Evaluation of Semisolid Jelly Produced by Musa acuminata Colla (AAA Group) Peels. Asian Pac. J. Trop. Biomed. 2016, 6, 26–31. [Google Scholar] [CrossRef]
- Schiassi, M.C.E.V.; Salgado, D.L.; Meirelles, B.S.; Lago, A.M.T.; Queiroz, F.; Curi, P.N.; Pio, R.; de Souza, V.R. Berry Jelly: Optimization Through Desirability-Based Mixture Design. J. Food Sci. 2019, 84, 1522–1528. [Google Scholar] [CrossRef]
- Lim, J.-H.; Park, S.-S.; Jeong, J.-W.; Park, K.-J.; Seo, K.-H.; Sung, J.-M. Quality Characteristics of Kimchi Fermented with Abalone or Sea Tangle Extracts. J. Korean Soc. Food Sci. Nutr. 2013, 42, 450–456. [Google Scholar] [CrossRef] [Green Version]
- Harwanto, D.; Saputro, P.; Susilowati, T.; Haditomo, A.H.C.; Windarto, S. Effect of Different n:P Ratios Application on the Cultivation Media for the Growth and Fiber Content of Caulerpa racemosa Reared in Tarpaulin Ponds. AACL Bioflux 2020, 13, 3117–3125. [Google Scholar]
- Everard, C.D.; O’Callaghan, D.J.; Fagan, C.C.; O’Donnell, C.P.; Castillo, M.; Payne, F.A. Computer Vision and Color Measurement Techniques for Inline Monitoring of Cheese Curd Syneresis. J. Dairy Sci. 2007, 90, 3162–3170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultrich, K.; Henderson, C.J.; Braeuning, A.; Buhrke, T. Correlation between 3-MCPD-Induced Organ Toxicity and Oxidative Stress Response in Male Mice. Food Chem. Toxicol. 2020, 136, 110957. [Google Scholar] [CrossRef] [PubMed]
- Sahadeva, R.P.K.; Leong, S.F.; Chua, K.H.; Tan, C.H.; Chan, H.Y.; Tong, E.V.; Wong, S.Y.W.; Chan, H.K. Survival of Commercial Probiotic Strains to PH and Bile. Int. Food Res. J. 2011, 18, 1515–1522. [Google Scholar]
- Rault, A.; Bouix, M.; Béal, C. Fermentation PH Influences the Physiological-State Dynamics of Lactobacillus bulgaricus CFL1 during PH-Controlled Culture. Appl. Environ. Microbiol. 2009, 75, 4374–4381. [Google Scholar] [CrossRef] [Green Version]
- Pasolli, E.; De Filippis, F.; Mauriello, I.E.; Cumbo, F.; Leech, J.; Cotter, P.D.; Segata, N.; Ercolini, D.; Walsh, A.M. Bacteria from Food With the Gut Microbiome. Nat. Commun. 2020, 11, 2610. [Google Scholar] [CrossRef] [PubMed]
- Ventura, E.E.; Davis, J.N.; Goran, M.I. Sugar Content of Popular Sweetened Beverages Based on Objective Laboratory Analysis: Focus on Fructose Content. Obesity 2011, 19, 868–874. [Google Scholar] [CrossRef]
- Featherstone, S. Jams, Jellies, and Related Products. In A Complete Course in Canning and Related Processes; Woodhead Publishing: Cambridgeshire, UK, 2016. [Google Scholar] [CrossRef]
- Stanhope, K.L. Sugar Consumption, Metabolic Disease and Obesity: The State of the Controversy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vera, M.; Dutta, B.; Mercer, D.G.; Maclean, H.L.; Touchie, M.F. Assessment of Moisture Content Measurement Methods of Dried Food Products in Small-Scale Operations in Developing Countries: A Review. Trends Food Sci. Technol. 2019, 88, 484–496. [Google Scholar] [CrossRef]
- Soedirga, L.C.; Marchellin, M. Physicochemical Properties of Jelly Candy Made with Pectin from Red Dragon Fruit Peel in Combination with Carrageenan. Caraka Tani J. Sustain. Agric. 2021, 37, 1–14. [Google Scholar] [CrossRef]
- Faridah, A. The Effect of Water Extract of Brown Seaweed on the Characteristic of Jelly Candy as a Functional Food. Int. J. Res. Rev. 2019, 6, 148–152. [Google Scholar]
- Hwang, E.-S.; Moon, S.J. Quality Characteristics and Antioxidant Activity of Stick Jelly Made with Different Amount of Tomato Juice. J. Korean Soc. Food Sci. Nutr. 2021, 50, 476–482. [Google Scholar] [CrossRef]
- Setiaboma, W.; Fitriani, V.; Mareta, D.T. Characterization of Fruit Leather with Carrageenan Addition with Various Bananas. IOP Conf. Ser. Earth Environ. Sci. 2019, 258, 012004. [Google Scholar] [CrossRef]
- Amin, P.; Riyadi, P.H.; Kurniasih, R.A.; Husni, A. Utilization of κ-Carrageenan as Stabilizer and Thickener of Honey Pineapple (Ananas Comosus [L. Merr]) Jam. Food Res. 2022, 6, 93–98. [Google Scholar] [CrossRef]
- Yusof, N.; Jaswir, I.; Jamal, P.; Jami, M.S. Texture Profile Analysis (TPA) of the Jelly Dessert Prepared from Halal Gelatin Extracted Using High Pressure Processing (HPP). Malays. J. Fundam. Appl. Sci. 2019, 15, 604–608. [Google Scholar] [CrossRef]
- Giger, A. Chemical Synthesis Project. A New Yellow Carotenoid. Pure Appl. Chem. 2002, 74, 1383–1390. [Google Scholar] [CrossRef] [Green Version]
- Paul, N.A.; Neveux, N.; Magnusson, M.; de Nys, R. Comparative Production and Nutritional Value of “Sea Grapes”—The Tropical Green Seaweeds Caulerpa lentillifera and C. racemosa. J. Appl. Phycol. 2014, 26, 1833–1844. [Google Scholar] [CrossRef]
- Potera, C. Forum the Artificial Food Dye Blues Invasion of the Bedbugs. Environ. Health Perspect. 2010, 108, 428–431. [Google Scholar] [CrossRef]
- Smejkal, Q.; Fiedler, T.; Kurz, T.; Kroh, L. Simplified Kinetics and Colour Formation in Sucrose Solutions Based on A-Dicarbonyl Compounds. Int. J. Food Eng. 2007, 3, 8. [Google Scholar] [CrossRef]
- Teixeira-Lemos, E.; Almeida, A.R.; Vouga, B.; Morais, C.; Correia, I.; Pereira, P.; Guiné, R.P.F. Development and Characterization of Healthy Gummy Jellies Containing Natural Fruits. Open Agric. 2021, 6, 466–478. [Google Scholar] [CrossRef]
- Satokari, R. High Intake of Sugar and the Balance between Pro- and Anti-Inflammatory Gut Bacteria. Nutrients 2020, 12, 1348. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Y.; Yu, H.; Chen, Z.; Tian, H. Influence of 4 Lactic Acid Bacteria on the Flavor Profile of Fermented Apple Juice Influence of 4 Lactic Acid Bacteria. Food Biosci. 2019, 27, 30–36. [Google Scholar] [CrossRef]
- Surini, S.; Diandra, D.M. Formulation of Mulberry Leaf (Morus Alba L.) Extract Hydrogel Beads Using Cross-Linked Pectin. Int. J. Appl. Pharm. 2017, 9, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alasalvar, C.; Pelvan, E.; Özdemir, K.S.; Kocadağlı, T.; Mogol, B.A.; Paslı, A.A.; Özcan, N.; Özçelik, B.; Gökmen, V. Compositional, Nutritional, and Functional Characteristics of Instant Teas Produced from Low- and High-Quality Black Teas. J. Agric. Food Chem. 2013, 61, 7529–7536. [Google Scholar] [CrossRef] [PubMed]
- Stenner, R.; Matubayasi, N.; Shimizu, S. Gelation of Carrageenan: Effects of Sugars and Polyols. Food Hydrocoll. 2016, 54, 284–292. [Google Scholar] [CrossRef]
- Watase, M.; Nishinari, K. Differential Scanning Calorimetry and Large Deformation Behaviour of Kappa-Carrageenan Gels Containing Alkali Metal Ions. Colloid Polym. Sci. 1985, 263, 744–748. [Google Scholar] [CrossRef]
- Tunnarut, D.; Pongsawatmanit, R. Quality Enhancement of Tapioca Starch Gel Using Sucrose and Xanthan Gum. Int. J. Food Eng. 2017, 13, 8. [Google Scholar] [CrossRef]
- Choi, D.H.; Kim, N.A.; Nam, T.S.; Lee, S.; Jeong, S.H. Evaluation of Taste-Masking Effects of Pharmaceutical Sweeteners with an Electronic Tongue System. Drug Dev. Ind. Pharm. 2014, 40, 308–317. [Google Scholar] [CrossRef]
- Kadota, K.; Nogami, S.; Uchiyama, H.; Tozuka, Y. Controlled Release Behavior of Curcumin from Kappa-Carrageenan Gels with Flexible Texture by the Addition of Metal Chlorides. Food Hydrocoll. 2020, 101, 105564. [Google Scholar] [CrossRef]
- Harada, T.; Uchida, T.; Yoshida, M.; Kobayashi, Y.; Narazaki, R.; Ohwaki, T. A New Method for Evaluating the Bitterness of Medicines in Development Using a Taste Sensor and a Disintegration Testing Apparatus. Chem. Pharm. Bull. 2010, 58, 1009–1014. [Google Scholar] [CrossRef] [Green Version]
- Ito, M.; Ikehama, K.; Yoshida, K.; Haraguchi, T.; Yoshida, M.; Wada, K.; Uchida, T. Bitterness Prediction of H1-Antihistamines and Prediction of Masking Effects of Artificial Sweeteners Using an Electronic Tongue. Int. J. Pharm. 2013, 441, 121–127. [Google Scholar] [CrossRef]
- Uno, R.; Ohkawa, K.; Kojima, H.; Haraguchi, T.; Ozeki, M.; Kawasaki, I.; Yoshida, M.; Habara, M.; Ikezaki, H.; Uchida, T. Masking the Taste of Fixed-Dose Combination Drugs: Particular NSAIDs Can Efficiently Mask the Bitterness of Famotidine. Chem. Pharm. Bull. 2023, 71, 148–153. [Google Scholar] [CrossRef]
- Takagi, A.; Kubo, R.; Jibiki, A.; Aomori, T.; Suzuki, S.; Nakamura, T. Identification of Foods Masking Clindamycin Bitterness Using an Electronic Gustatory Screening System Followed by Organoleptic Examination. Iryo Yakugaku (Jpn. J. Pharm. Health Care Sci.) 2017, 43, 492–501. [Google Scholar] [CrossRef] [Green Version]
- Sutriyo, S.; Iswandana, R.; Fauzi, F. Strategy to Mask the Bitter Taste of Momordica Charantia Extract Using Alginate–Gelatin Beads. Int. J. Appl. Pharm. 2018, 10, 381. [Google Scholar] [CrossRef] [Green Version]
- Bin, L.K.; Gaurav, A.; Mandal, U.K. A Review on Co-Processed Excipients: Current and Future Trend of Excipient Technology. Int. J. Pharm. Pharm. Sci. 2019, 11, 1. [Google Scholar] [CrossRef]
- Kuhn, C.; Bufe, B.; Winnig, M.; Hofmann, T.; Frank, O.; Behrens, M.; Lewtschenko, T.; Slack, J.P.; Ward, C.D.; Meyerhof, W. Bitter Taste Receptors for Saccharin and Acesulfame K. J. Neurosci. 2004, 24, 10260–10265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, Y.; Kiuchi, H.; Haraguchi, T.; Yoshida, M.; Uchida, T.; Hanawa, T. Preparation and Evaluation of Physicochemical Propertiesof Isosorbide Gel Composed of Xanthan Gum, Locust Bean Gum and Agar for Improving the Patients Adherence. Int. J. Med. Pharm. 2017, 5, 18–32. [Google Scholar] [CrossRef] [Green Version]
- Kadam, V.S.; Kendre, J.; Shendarkar, G.R.; Kadam, S.S. Formulation and Evaluation of Medicated Oral Jelly of Trazadone Hydrochloride. Int. J. Pharm. Sci. Res. 2020, 11, 6251. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Habara, M.; Ikezazki, H.; Chen, R.; Naito, Y.; Toko, K. Advanced Taste Sensors Based on Artificial Lipids with Global Selectivity to Basic Taste Qualities and High Correlation to Sensory Scores. Sensors 2010, 10, 3411–3443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimenta Pereira, P.A.; de Souza, V.R. Influence of gelling agent concentration on the characteristics of functional sugar-free guava preserves. Emir. J. Food Agric. 2019, 31, 501–510. [Google Scholar] [CrossRef]
 is the independent variables data, while
is the independent variables data, while  is missing independent variables.
is missing independent variables.
 is the independent variables data, while
is the independent variables data, while  is missing independent variables.
is missing independent variables.





| No. | A | B | C | D | E | F | G | pH | TSS, % | MS, % |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12.50 | 24.89 | 21.63 | 26 | 13 | 1.95 | 0.03 | 5.94 | 45.1 | 38.7 |
| 2 | 15.00 | 22.03 | 22.02 | 26 | 13 | 1.95 | 0 | 5.97 | 41.5 | 41.3 |
| 3 | 13.75 | 21.63 | 23.66 | 26 | 13 | 1.95 | 0.01 | 5.96 | 48.0 | 42.9 |
| 4 | 12.50 | 23.28 | 23.27 | 26 | 13 | 1.95 | 0 | 5.93 | 47.4 | 35.4 |
| 5 | 12.50 | 20.00 | 26.55 | 26 | 13 | 1.95 | 0 | 5.91 | 48.5 | 36.9 |
| 6 | 15.00 | 24.00 | 20.00 | 26 | 13 | 1.95 | 0.05 | 6.20 | 43.1 | 49.3 |
| 7 | 12.50 | 26.53 | 20.00 | 26 | 13 | 1.95 | 0.02 | 5.93 | 45.6 | 40.4 |
| 8 | 10.00 | 20.00 | 29.00 | 26 | 13 | 1.95 | 0.05 | 5.83 | 48.8 | 39.9 |
| 9 | 10.00 | 29.05 | 20.00 | 26 | 13 | 1.95 | 0 | 5.87 | 50.1 | 35.6 |
| 10 | 10.00 | 24.51 | 24.51 | 26 | 13 | 1.95 | 0.03 | 5.83 | 49.7 | 30.4 |
| 11 | 15.00 | 24.05 | 20.00 | 26 | 13 | 1.95 | 0 | 6.10 | 45.0 | 42.5 |
| 12 | 15.00 | 24.05 | 20.00 | 26 | 13 | 1.95 | 0 | 5.98 | 44.5 | 43.1 |
| 13 | 10.00 | 20.00 | 29.05 | 26 | 13 | 1.95 | 0 | 5.88 | 48.7 | 32.7 |
| 14 | 15.00 | 20.00 | 24.03 | 26 | 13 | 1.95 | 0.02 | 5.98 | 44.5 | 47.7 |
| 15 | 10.00 | 29.00 | 20.00 | 26 | 13 | 1.95 | 0.05 | 5.82 | 51.8 | 38.7 |
| 16 | 10.00 | 29.00 | 20.00 | 26 | 13 | 1.95 | 0.05 | 5.84 | 52.5 | 39.2 |
| 17 | 11.25 | 21.63 | 26.13 | 26 | 13 | 1.95 | 0.04 | 5.87 | 45.5 | 38.0 |
| Sources | Physical Parameters | ||
|---|---|---|---|
| pH | TSS, % | MS, % | |
| Model | Significant | Significant | Significant |
| R2 | 0.9941 | 0.9907 | 0.9980 |
| Adjusted R2 | 0.9836 | 0.9699 | 0.9934 |
| Predicted R2 | 0.9354 | 0.9344 | 0.9226 |
| Adequate Precision | 38.2014 | 25.0094 | 50.4348 |
| p-value | <0.0001 | 0.0010 | <0.0001 |
| F value | 94.31 | 47.49 | 217.37 |
| C.V. % | 0.21 | 0.83 | 1.04 |
| Standard deviation | 0.0123 | 0.3880 | 0.3955 |
| PRESS | 0.0083 | 4.26 | 0.9980 |
| Independent Variables | Responses | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | TSS | MS | ||||||||||||||
| No. | A | B | C | D | E | F | G | X | Y | RSE | X | Y | RSE | X | Y | RSE |
| 1 | 14.3 | 23.0 | 21.7 | 26.0 | 13.0 | 1.9 | 0 | 5.97 | 5.96 | 0.17 | 45.8 | 44.8 | 2.18 | 40.0 | 38.4 | 4.00 |
| 2 | 14.1 | 23.0 | 21.8 | 26.0 | 13.0 | 1.9 | 0 | 5.96 | 5.93 | 0.50 | 45.9 | 46.8 | 1.96 | 39.5 | 37.8 | 4.30 |
| 3 | 14.1 | 23.5 | 21.3 | 26.0 | 13.0 | 1.9 | 0 | 5.97 | 5.94 | 0.51 | 46.3 | 46.8 | 1.07 | 39.9 | 38.2 | 4.26 |
| Sample/Sugar | Fructose | Glucose | Sucrose | Maltose | Total, % |
|---|---|---|---|---|---|
| Jelly Run 4 | 0.22 ± 0.01 | 4.19 ± 0.054 | 25.17 ± 0.48 | 2.77 ± 0.09 | 32.37 |
| Jelly Run 10 | 0.18 ± 0.00 | 4.52 ± 0.062 | 26.91 ± 0.42 | 3.01 ± 0.72 | 34.63 |
| Jelly Run 12 | 0.20 ± 0.035 | 3.89 ± 0.074 | 24.35 ± 1.21 | 2.55 ± 0.06 | 31.00 |
| Control | ND | 4.72 ± 0.035 | 29.76 ± 0.473 | 3.22 ± 0.13 | 37.73 |
| Extract | 3.687 ± 0.04 | 3.754 ± 0.023 | ND | ND | 7.37 |
| Commercial jelly | 1.23 ± 0.00 | 11.937 ± 0.00 | 24.658 ± 0.07 | 5.70 ± 0.16 | 43.53 |
| p-value | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Causal Factor Variables | Coded Level of Variable (%) | |
|---|---|---|
| Low (−1) | High (+1) | |
| Extract | 10 | 15 |
| Sucrose | 20 | 29.05 |
| Fructose | 20 | 29.05 |
| Methylparaben | 0 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasir, N.A.H.A.; Yuswan, M.H.; Shah, N.N.A.K.; Abd Rashed, A.; Kadota, K.; Yusof, Y.A. Evaluation of Physicochemical Properties of a Hydrocolloid-Based Functional Food Fortified with Caulerpa lentillifera: A D-Optimal Design Approach. Gels 2023, 9, 531. https://doi.org/10.3390/gels9070531
Nasir NAHA, Yuswan MH, Shah NNAK, Abd Rashed A, Kadota K, Yusof YA. Evaluation of Physicochemical Properties of a Hydrocolloid-Based Functional Food Fortified with Caulerpa lentillifera: A D-Optimal Design Approach. Gels. 2023; 9(7):531. https://doi.org/10.3390/gels9070531
Chicago/Turabian StyleNasir, Nor Atikah Husna Ahmad, Mohd Hafis Yuswan, Nor Nadiah Abd Karim Shah, Aswir Abd Rashed, Kazunori Kadota, and Yus Aniza Yusof. 2023. "Evaluation of Physicochemical Properties of a Hydrocolloid-Based Functional Food Fortified with Caulerpa lentillifera: A D-Optimal Design Approach" Gels 9, no. 7: 531. https://doi.org/10.3390/gels9070531