Propranolol-Loaded Limonene-Based Microemulsion Thermo-Responsive Mucoadhesive Nasal Nanogel: Design, In Vitro Assessment, Ex Vivo Permeation, and Brain Biodistribution
Abstract
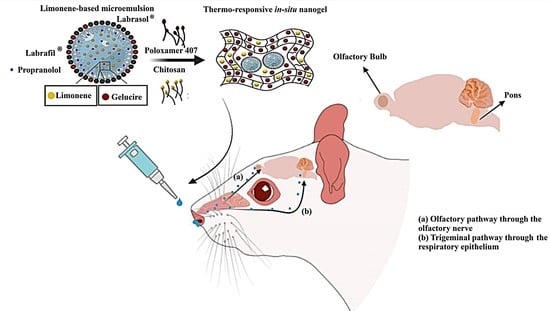
:1. Introduction
2. Results and Discussion
2.1. Fabrication and Characterization of Propranolol-Loaded Limonene-Based Microemulsion
2.2. Fabrication and Characterization of Thermo-Responsive Nanogel
2.3. In-Vitro Release and Ex-Vivo Permeation
2.4. In-Vivo Evaluation
2.4.1. Histopathological Examination
2.4.2. Brain Uptake Study
2.5. Stability Study
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Fabrication and Characterization of Propranolol-Loaded Limonene-Based Microemulsion System (PRO-ME)
4.2.2. Fabrication of Thermo-Responsive Nanogel
4.2.3. Preparation and Characterization of Thermo-Responsive Nanogel
Sol–Gel Transition Temperature, Gelation Time, and Gelation Strength
pH, Viscosity, and Drug Content Estimation
Mucoadhesive Strength
In-Vitro Release Study
4.2.4. Ex-Vivo Permeation Study
Preparation of Sheep Nasal Mucosa
Intranasal Propranolol Delivery
4.2.5. Assessment of Local Toxicity of the In Situ Nanogel through Histopathological Examination
4.2.6. Brain Uptake Study
Experimental Animal
Intranasal and Oral Administration
Extraction of Propranolol from Brain Tissues
HPLC Analysis
Brain Pharmacokinetics
4.2.7. Stability Study
4.2.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sim, T.M.; Tarini, D.; Dheen, S.T.; Bay, B.H.; Srinivasan, D.K. Nanoparticle-Based Technology Approaches to the Management of Neurological Disorders. Int. J. Mol. Sci. 2020, 21, 6070. [Google Scholar] [CrossRef]
- Carroll, W.M. The Global Burden of Neurological Disorders. Lancet Neurol. 2019, 18, 418–419. [Google Scholar] [CrossRef] [Green Version]
- Charles, A. The Pathophysiology of Migraine: Implications for Clinical Management. Lancet Neurol. 2018, 17, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Tepper, S.J.; Lucas, S.; Rasmussen, S.; Nelson, R. A Narrative Review of the Importance of Pharmacokinetics and Drug–Drug Interactions of Preventive Therapies in Migraine Management. Headache 2021, 61, 838–853. [Google Scholar] [CrossRef]
- Danesh, A.; Gottschalk, P.C.H. Beta-Blockers for Migraine Prevention: A Review Article. Curr. Treat. Options Neurol. 2019, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.; Tomić, N.; Cvijić, S.; Stojanović, D.; Ibrić, S.; Uskoković, P. Mucoadhesive Gelatin Buccal Films with Propranolol Hydrochloride: Evaluation of Mechanical, Mucoadhesive, and Biopharmaceutical Properties. Pharmaceutics 2021, 13, 273. [Google Scholar] [CrossRef]
- Mohamad, S.A.; Salem, H.; Yassin, H.A.; Mansour, H.F. Bucco-Adhesive Film as a Pediatric Proper Dosage Form for Systemic Delivery of Propranolol Hydrochloride: In-Vitro and in-Vivo Evaluation. Drug Des. Devel. Ther. 2020, 14, 4277–4289. [Google Scholar] [CrossRef]
- Agrawal, M.; Saraf, S.; Saraf, S.; Dubey, S.K.; Puri, A.; Gupta, U.; Kesharwani, P.; Ravichandiran, V.; Kumar, P.; Naidu, V.G.M.; et al. Stimuli-Responsive in Situ Gelling System for Nose-to-Brain Drug Delivery. J. Control. Release 2020, 327, 235–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, G.; Ma, J.; Guo, S.; Gao, L.; Jia, Y.; Li, X.; Zhang, Q. In Situ Gel-Forming System: An Attractive Alternative for Nasal Drug Delivery. Crit. Rev. Ther. Drug Carr. Syst. 2013, 30, 411–434. [Google Scholar] [CrossRef]
- Jagdale, S.; Shewale, N.; Kuchekar, B.S. Optimization of Thermoreversible in Situ Nasal Gel of Timolol Maleate. Scientifica 2016, 2016, 6401267. [Google Scholar] [CrossRef] [Green Version]
- Mehanna, M.M.; Mneimneh, A.T.; Abed El Jalil, K. Levofloxacin-Loaded Naturally Occurring Monoterpene-Based Nanoemulgel: A Feasible Efficient System to Circumvent MRSA Ocular Infections. Drug Dev. Ind. Pharm. 2020, 46, 1787–1799. [Google Scholar] [CrossRef]
- Liao, A.H.; Shih, C.P.; Li, M.W.; Lin, Y.C.; Chuang, H.C.; Wang, C.H. Development of Thermosensitive Poloxamer 407-Based Microbubble Gel with Ultrasound Mediation for Inner Ear Drug Delivery. Drug Deliv. 2021, 28, 1256–1271. [Google Scholar] [CrossRef]
- Fathalla, Z.; Mustafa, W.W.; Abdelkader, H.; Moharram, H.; Sabry, A.M.; Alany, R.G. Hybrid Thermosensitive-Mucoadhesive in Situ Forming Gels for Enhanced Corneal Wound Healing Effect of L-Carnosine. Drug Deliv. 2022, 29, 374–385. [Google Scholar] [CrossRef]
- Elkomy, M.H.; Ali, A.A.; Eid, H.M. Chitosan on the Surface of Nanoparticles for Enhanced Drug Delivery: A Comprehensive Review. J. Control. Release 2022, 351, 923–940. [Google Scholar] [CrossRef]
- Trevino, J.T.; Quispe, R.C.; Khan, F.; Novak, V. Non-Invasive Strategies for Nose-to-Brain Drug Delivery. J. Clin. Trials 2020, 10, 439. [Google Scholar]
- Froelich, A.; Osmałek, T.; Jadach, B.; Puri, V.; Michniak-Kohn, B. Microemulsion-Based Media in Nose-to-Brain Drug Delivery. Pharmaceutics 2021, 13, 201. [Google Scholar] [CrossRef]
- Thakur, D.; Kaur, G.; Puri, A.; Nanda, R. Therapeutic Potential of Essential Oil Based Microemulsions: Reviewing State-of-the-Art. Curr. Drug Deliv. 2021, 18, 1218–1233. [Google Scholar] [CrossRef]
- Eddin, L.B.; Jha, N.K.; Meeran, M.F.N.; Kesari, K.K.; Beiram, R.; Ojha, S. Neuroprotective Potential of Limonene and Limonene Containing Natural Products. Molecules 2021, 4535, 4535. [Google Scholar] [CrossRef]
- Piccinelli, A.C.; Morato, P.N.; Dos Santos Barbosa, M.; Croda, J.; Sampson, J.; Kong, X.; Konkiewitz, E.C.; Ziff, E.B.; Amaya-Farfan, J.; Kassuya, C.A.L. Limonene Reduces Hyperalgesia Induced by Gp120 and Cytokines by Modulation of IL-1 β and Protein Expression in Spinal Cord of Mice. Life Sci. 2017, 174, 28–34. [Google Scholar] [CrossRef]
- Mehanna, M.M.; Abla, K.K. SiRNA Nanohybrid Systems: False Hope or Feasible Answer in Cancer Management. Ther. Deliv. 2022, 13, 109–133. [Google Scholar] [CrossRef]
- Alwattar, J.K.; Mneimneh, A.T.; Abla, K.K.; Mehanna, M.M.; Allam, A.N. Smart Stimuli-Responsive Liposomal Nanohybrid Systems: A Critical Review of Theranostic Behavior in Cancer. Pharmaceutics 2021, 13, 355. [Google Scholar] [CrossRef]
- Abla, K.K.; Mneimneh, A.T.; Allam, A.N.; Mehanna, M.M. Application of Box-Behnken Design in the Preparation, Optimization, and In-Vivo Pharmacokinetic Evaluation of Oral Tadalafil-Loaded Niosomal Film. Pharmaceutics 2023, 15, 173. [Google Scholar] [CrossRef]
- Nayak, K.; Choudhari, M.V.; Bagul, S.; Chavan, T.A.; Misra, M. Ocular Drug Delivery Systems. In Developments in Biomedical Engineering and Bioelectronics; Academic Press: Cambridge, MA, USA, 2021; pp. 515–566. ISBN 25897527. [Google Scholar]
- Agatonovic-Kustrin, S.; Chan, C.K.Y.; Gegechkori, V.; Morton, D.W. Models for Skin and Brain Penetration of Major Components from Essential Oils Used in Aromatherapy for Dementia Patients. J. Biomol. Struct. Dyn. 2020, 38, 2402–2411. [Google Scholar] [CrossRef]
- Gorantla, S.; Rapalli, V.K.; Waghule, T.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Nanocarriers for Ocular Drug Delivery: Current Status and Translational Opportunity. RSC Adv. 2020, 10, 27835–27855. [Google Scholar] [CrossRef]
- Wang, X.; Cui, X.; Zhao, Y.; Chen, C. Nano-Bio Interactions: The Implication of Size-Dependent Biological Effects of Nanomaterials. Sci. China Life Sci. 2020, 63, 1168–1182. [Google Scholar] [CrossRef]
- Clares, B.; Calpena, A.; Parra, A.; Abrego, G.; Alvarado, H.; Fangueiro, J.; Souto, E. Nanoemulsions (NEs), Liposomes (LPs) and Solid Lipid Nanoparticles (SLNs) for Retinyl Palmitate: Effect on Skin Permeation. Int. J. Pharm. 2014, 473, 591–598. [Google Scholar] [CrossRef]
- Katare, Y.K.; Daya, R.P.; Sookram Gray, C.; Luckham, R.E.; Bhandari, J.; Chauhan, A.S.; Mishra, R.K. Brain Targeting of a Water Insoluble Antipsychotic Drug Haloperidol via the Intranasal Route Using PAMAM Dendrimer. Mol. Pharm. 2015, 12, 3380–3388. [Google Scholar] [CrossRef] [Green Version]
- Sherif, S.; Bendas, E.; Badawy, S. The Clinical Efficacy of Cosmeceutical Application of Liquid Crystalline Nanostructured Dispersions of Alpha Lipoic Acid as Anti-Wrinkle. Eur. J. Pharm. Biopharm. 2014, 86, 251–259. [Google Scholar] [CrossRef]
- Mohyeldin, S.M.; Mehanna, M.M.; Elgindy, N.A. Superiority of Liquid Crystalline Cubic Nanocarriers as Hormonal Transdermal Vehicle: Comparative Human Skin Permeation-Supported Evidence. Expert Opin. Drug Deliv. 2016, 13, 1049–1064. [Google Scholar] [CrossRef]
- Sis, H.; Birinci, M. Effect of Nonionic and Ionic Surfactants on Zeta Potential and Dispersion Properties of Carbon Black Powders. Colloids Surfaces A Physicochem. Eng. Asp. 2009, 341, 60–67. [Google Scholar] [CrossRef]
- Lin, W.; Kampf, N.; Goldberg, R.; Driver, M.J.; Klein, J. Poly-Phosphocholinated Liposomes Form Stable Superlubrication Vectors. Langmuir 2019, 35, 6048–6054. [Google Scholar] [CrossRef]
- Wulff-Pérez, M.; de Vicente, J.; Martín-Rodríguez, A.; Gálvez-Ruiz, M.J. Controlling Lipolysis through Steric Surfactants: New Insights on the Controlled Degradation of Submicron Emulsions after Oral and Intravenous Administration. Int. J. Pharm. 2012, 423, 161–166. [Google Scholar] [CrossRef]
- Shewaiter, M.A.; Hammady, T.M.; El-Gindy, A.; Hammadi, S.H.; Gad, S. Formulation and Characterization of Leflunomide/Diclofenac Sodium Microemulsion Base-Gel for the Transdermal Treatment of Inflammatory Joint Diseases. J. Drug Deliv. Sci. Technol. 2021, 61, 102110. [Google Scholar] [CrossRef]
- Li, Y.; Ruan, S.; Wang, Z.; Feng, N.; Zhang, Y. Hyaluronic Acid Coating Reduces the Leakage of Melittin Encapsulated in Liposomes and Increases Targeted Delivery to Melanoma Cells. Pharmaceutics 2021, 13, 1235. [Google Scholar] [CrossRef]
- Subongkot, T.; Ngawhirunpat, T. Development of a Novel Microemulsion for Oral Absorption Enhancement of All-Trans Retinoic Acid. Int. J. Nanomedicine 2017, 12, 5585–5599. [Google Scholar] [CrossRef] [Green Version]
- Meka, V.S.; Nali, S.R.; Songa, A.S.; Battu, J.R.; Kolapalli, V.R.M. Statistical Optimization of a Novel Excipient (CMEC) Based Gastro Retentive Floating Tablets of Propranolol HCl and Its in Vivo Buoyancy Characterization in Healthy Human Volunteers. DARU J. Pharm. Sci. 2012, 20, 21. [Google Scholar] [CrossRef] [Green Version]
- Irimia, T.; Dinu-Pîrvu, C.E.; Ghica, M.V.; Lupuleasa, D.; Muntean, D.L.; Udeanu, D.I.; Popa, L. Chitosan-Based in Situ Gels for Ocular Delivery of Therapeutics: A State-of-the-Art Review. Mar. Drugs 2018, 16, 373. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, T.A.; El-Say, K.M.; Ahmed, O.A.A.; Aljaeid, B.M. Superiority of Tpgs-Loaded Micelles in the Brain Delivery of Vinpocetine via Administration of Thermosensitive Intranasal Gel. Int. J. Nanomed. 2019, 14, 5555–5567. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Gao, Q.; Lu, X.; Zhou, H. In Situ Forming Hydrogels Based on Chitosan for Drug Delivery and Tissue Regeneration. Asian J. Pharm. Sci. 2016, 11, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Sindhoor, S.M.; Priya, S.; Maxwell, A. Formulation and Evaluation of Novel in Situ Gel of Lafutidine for Gastro Retentive Drug Delivery. Asian J. Pharm. Clin. Res. 2018, 11, 88. [Google Scholar] [CrossRef]
- Mura, P.; Mennini, N.; Nativi, C.; Richichi, B. In Situ Mucoadhesive-Thermosensitive Liposomal Gel as a Novel Vehicle for Nasal Extended Delivery of Opiorphin. Eur. J. Pharm. Biopharm. 2018, 122, 54–61. [Google Scholar] [CrossRef]
- Kaur, P.; Garg, T.; Rath, G.; Goyal, A.K. In Situ Nasal Gel Drug Delivery: A Novel Approach for Brain Targeting through the Mucosal Membrane. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; Badr-Eldin, S.M.; Ahmed, O.A.A.; Aldawsari, H. Intranasal Optimized Solid Lipid Nanoparticles Loaded in Situ Gel for Enhancing Trans-Mucosal Delivery of Simvastatin. J. Drug Deliv. Sci. Technol. 2018, 48, 499–508. [Google Scholar] [CrossRef]
- Pai, R.V.; Monpara, J.D.; Vavia, P.R. Exploring Molecular Dynamics Simulation to Predict Binding with Ocular Mucin: An in Silico Approach for Screening Mucoadhesive Materials for Ocular Retentive Delivery Systems. J. Control. Release 2019, 309, 190–202. [Google Scholar] [CrossRef]
- Patel, N.; Nakrani, H.; Raval, M.; Sheth, N. Development of Loteprednol Etabonate-Loaded Cationic Nanoemulsified in-Situ Ophthalmic Gel for Sustained Delivery and Enhanced Ocular Bioavailability. Drug Deliv. 2016, 23, 3712–3723. [Google Scholar] [CrossRef] [Green Version]
- Salade, L.; Wauthoz, N.; Goole, J.; Amighi, K. How to Characterize a Nasal Product. The State of the Art of in Vitro and Ex Vivo Specific Methods. Int. J. Pharm. 2019, 561, 47–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.-D. In Vitro Cellular Models for Nasal Drug Absorption Studies BT—Drug Absorption Studies: In Situ, In Vitro and In Silico Models; Ehrhardt, C., Kim, K.-J., Eds.; Springer US: Boston, MA, USA, 2008; pp. 216–234. ISBN 9780387749013. [Google Scholar]
- Gerber, W.; Svitina, H.; Steyn, D.; Peterson, B.; Kotzé, A.; Weldon, C.; Hamman, J.H. Comparison of RPMI 2650 Cell Layers and Excised Sheep Nasal Epithelial Tissues in Terms of Nasal Drug Delivery and Immunocytochemistry Properties. J. Pharmacol. Toxicol. Methods 2022, 113, 107131. [Google Scholar] [CrossRef]
- Tas, C.; Ozkan, Y.; Okyar, A.; Savaser, A. In Vitro and Ex Vivo Permeation Studies of Etodolac from Hydrophilic Gels and Effect of Terpenes as Enhancers. Drug Deliv. 2007, 14, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Bekhet, M.A.; Ali, A.A.; Kharshoum, R.M.; El-Ela, F.I.A.; Salem, H.F. Intranasal Niosomal In Situ Gel As A Novel Strategy for Improving Citicoline Efficacy and Brain Delivery in Treatment of Epilepsy: In Vitro and Ex Vivo Characterization and In Vivo Pharmacodynamics Investigation. J. Pharm. Sci. 2022, 111, 2258–2269. [Google Scholar] [CrossRef]
- Khan, K.; Aqil, M.; Imam, S.S.; Ahad, A.; Moolakkadath, T.; Sultana, Y.; Mujeeb, M. Ursolic Acid Loaded Intra Nasal Nano Lipid Vesicles for Brain Tumour: Formulation, Optimization, in-Vivo Brain/Plasma Distribution Study and Histopathological Assessment. Biomed. Pharmacother. 2018, 106, 1578–1585. [Google Scholar] [CrossRef]
- Waheed, S.; Li, Z.; Zhang, F.; Chiarini, A.; Armato, U.; Wu, J. Engineering Nano-Drug Biointerface to Overcome Biological Barriers toward Precision Drug Delivery. J. Nanobiotechnology 2022, 20, 395. [Google Scholar] [CrossRef]
- Younes, N.F.; Abdel-halim, S.A.; Elassasy, A.I. Corneal Targeted Sertaconazole Nitrate Loaded Cubosomes: Preparation, Statistical Optimization, in Vitro Characterization, Ex Vivo Permeation and in Vivo Studies. Int. J. Pharm. 2018, 553, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Bayanati, M.; Khosroshahi, A.G.; Alvandi, M.; Mahboobian, M.M. Fabrication of a Thermosensitive in Situ Gel Nanoemulsion for Nose to Brain Delivery of Temozolomide. J. Nanomater. 2021, 2021, 1546798. [Google Scholar] [CrossRef]
- Abdulla, N.A.; Balata, G.F.; El-ghamry, H.A.; Gomaa, E. Intranasal Delivery of Clozapine Using Nanoemulsion-Based in-Situ Gels: An Approach for Bioavailability Enhancement. Saudi Pharm. J. 2021, 29, 1466–1485. [Google Scholar] [CrossRef]
- Parashar, P.; Diwaker, N.; Kanoujia, J.; Singh, M.; Yadav, A.; Singh, I.; Saraf, S.A. In Situ Gel of Lamotrigine for Augmented Brain Delivery: Development Characterization and Pharmacokinetic Evaluation. J. Pharm. Investig. 2020, 50, 95–105. [Google Scholar] [CrossRef]
- Singh, A.P.; Saraf, S.K.; Saraf, S.A. SLN Approach for Nose-to-Brain Delivery of Alprazolam. Drug Deliv. Transl. Res. 2012, 2, 498–507. [Google Scholar] [CrossRef]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of Intranasal Drug Delivery Directly to the Brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Emad, N.A.; Ahmed, B.; Alhalmi, A.; Alzobaidi, N.; Al-Kubati, S.S. Recent Progress in Nanocarriers for Direct Nose to Brain Drug Delivery. J. Drug Deliv. Sci. Technol. 2021, 64, 102642. [Google Scholar] [CrossRef]
- Agrawal, M.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Chougule, M.B.; Shoyele, S.A.; Alexander, A. Nose-to-Brain Drug Delivery: An Update on Clinical Challenges and Progress towards Approval of Anti-Alzheimer Drugs. J. Control. Release 2018, 281, 139–177. [Google Scholar] [CrossRef]
- Ceña, V.; Játiva, P. Nanoparticle Crossing of Blood-Brain Barrier: A Road to New Therapeutic Approaches to Central Nervous System Diseases. Nanomedicine 2018, 13, 1513–1516. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; He, H.; Li, F.; Lu, Y.; Qi, J.; Wu, W. An Update on the Role of Nanovehicles in Nose-to-Brain Drug Delivery. Drug Discov. Today 2018, 23, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Formica, M.L.; Real, D.A.; Picchio, M.L.; Catlin, E.; Donnelly, R.F.; Paredes, A.J. On a Highway to the Brain: A Review on Nose-to-Brain Drug Delivery Using Nanoparticles. Appl. Mater. Today 2022, 29, 101631. [Google Scholar] [CrossRef]
- Huang, T.W.; Li, S.T.; Young, T.H. Chitosan-Hyaluronan: Promotion of Mucociliary Differentiation of Respiratory Epithelial Cells and Development of Olfactory Receptor Neurons. Artif. Cells Nanomed. Biotechnol. 2019, 47, 564–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.; Minko, T. Nanotherapeutics for Nose-to-Brain Drug Delivery: An Approach to Bypass the Blood Brain Barrier. Pharmaceutics 2021, 13, 2049. [Google Scholar] [CrossRef] [PubMed]
- Gabal, Y.M.; Kamel, A.O.; Sammour, O.A.; Elshafeey, A.H. Effect of Surface Charge on the Brain Delivery of Nanostructured Lipid Carriers in Situ Gels via the Nasal Route. Int. J. Pharm. 2014, 473, 442–457. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, M.; Zhang, J.; Maincent, P.; Xia, X.; Wu, W. Updated Progress of Nanocarrier-Based Intranasal Drug Delivery Systems for Treatment of Brain Diseases. Crit. Rev. Ther. Drug Carr. Syst. 2018, 35, 433–468. [Google Scholar] [CrossRef]
- Akhtar, N.; Ahad, A.; Khar, R.K.; Jaggi, M.; Aqil, M.; Iqbal, Z.; Ahmad, F.J.; Talegaonkar, S. The Emerging Role of P-Glycoprotein Inhibitors in Drug Delivery: A Patent Review. Expert Opin. Ther. Pat. 2011, 21, 561–576. [Google Scholar] [CrossRef]
- Mehanna, M.M.; Abla, K.K.; Elmaradny, H.A. Tailored Limonene-Based Nanosized Microemulsion: Formulation, Physicochemical Characterization and in-Vivo Skin Irritation Assessment. Adv. Pharm. Bull. 2020, 11, 274–285. [Google Scholar] [CrossRef]
- Mehanna, M.M.; Abla, K.K.; Domiati, S.; Elmaradny, H. Superiority of Microemulsion-Based Hydrogel for Non-Steroidal Anti-Inflammatory Drug Transdermal Delivery: A Comparative Safety and Anti-Nociceptive Efficacy Study. Int. J. Pharm. 2022, 622, 121830. [Google Scholar] [CrossRef]
- Rizwanullah, M.; Amin, S.; Ahmad, J. Improved Pharmacokinetics and Antihyperlipidemic Efficacy of Rosuvastatin-Loaded Nanostructured Lipid Carriers. J. Drug Target. 2017, 25, 58–74. [Google Scholar] [CrossRef]
- Xin, Y.; Yun, S.; Yuhe, L.; Yinxue, M.; Shurui, N.; Yue, Z.; Kunming, Q.; Weidong, L. Development of Licorice Flavonoids Loaded Microemulsion for Transdermal Delivery Using CCD-Optimal Experimental Approach: Formulation Development and Characterization. Front. Nanotechnol. 2021, 3, 748791. [Google Scholar] [CrossRef]
- Huang, W.; Dou, H.; Wu, H.; Sun, Z.; Wang, H.; Huang, L. Preparation and Characterisation of Nobiletin-Loaded Nanostructured Lipid Carriers. J. Nanomater. 2017, 2017, 2898342. [Google Scholar] [CrossRef] [Green Version]
- Eldeeb, A.E.; Salah, S.; Ghorab, M. Formulation and Evaluation of Cubosomes Drug Delivery System for Treatment of Glaucoma: Ex-Vivo Permeation and in-Vivo Pharmacodynamic Study. J. Drug Deliv. Sci. Technol. 2019, 52, 236–247. [Google Scholar] [CrossRef]
- Khan, A.; Aqil, M.; Imam, S.S.; Ahad, A.; Sultana, Y.; Ali, A.; Khan, K. Temozolomide Loaded Nano Lipid Based Chitosan Hydrogel for Nose to Brain Delivery: Characterization, Nasal Absorption, Histopathology and Cell Line Study. Int. J. Biol. Macromol. 2018, 116, 1260–1267. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Ahmad, F.J.; Ahmad, W.; Alam, M.A.; Amir, M.; Ali, A. Poloxamer-Chitosan-Based Naringenin Nanoformulation Used in Brain Targeting for the Treatment of Cerebral Ischemia. Saudi J. Biol. Sci. 2020, 27, 500–517. [Google Scholar] [CrossRef]
- Hosny, K.M.; Rizg, W.Y.; Khallaf, R.A. Preparation and Optimization of in Situ Gel Loaded with Rosuvastatin-Ellagic Acid Nanotransfersomes to Enhance the Anti-Proliferative Activity. Pharmaceutics 2020, 12, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saudagar, R.B.; Khandbahale, S.V. Formulation Development and Evaluation of Nasal In-Situ Gel of Fluticasone Propionate. Int. J. Curr. Pharm. Res. 2017, 9, 45–54. [Google Scholar] [CrossRef]
- Hosny, K.M.; Hassan, A.H. Intranasal in Situ Gel Loaded with Saquinavir Mesylate Nanosized Microemulsion: Preparation, Characterization, and in Vivo Evaluation. Int. J. Pharm. 2014, 475, 191–197. [Google Scholar] [CrossRef]
- Mandal, S.; Thimmasetty, M.K.; Prabhushankar, G.; Geetha, M. Formulation and Evaluation of an in Situ Gel-Forming Ophthalmic Formulation of Moxifloxacin Hydrochloride. Int. J. Pharm. Investig. 2012, 2, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.B.; Chaudhary, S.; Jacob, S.; Patel, D.; Shinu, P.; Shah, H.; Chaudhary, A.; Aldhubiab, B.; Almuqbil, R.M.; Alnaim, A.S.; et al. Intranasal Administration of Dolutegravir-Loaded Nanoemulsion-Based In Situ Gel for Enhanced Bioavailability and Direct Brain Targeting. Gels 2023, 9, 130. [Google Scholar] [CrossRef]
- Pund, S.; Rasve, G.; Borade, G. Ex Vivo Permeation Characteristics of Venlafaxine through Sheep Nasal Mucosa. Eur. J. Pharm. Sci. 2013, 48, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Galgatte, U.C.; Kumbhar, A.B.; Chaudhari, P.D. Development of in Situ Gel for Nasal Delivery: Design, Optimization, in Vitro and in Vivo Evaluation. Drug Deliv. 2014, 21, 62–73. [Google Scholar] [CrossRef] [Green Version]
- Fahmy, U.A.; Ahmed, O.A.A.; Badr-Eldin, S.M.; Aldawsari, H.M.; Okbazghi, S.Z.; Awan, Z.A.; Bakhrebah, M.A.; Alomary, M.N.; Abdulaal, W.H.; Medina, C.; et al. Development and Validation of HPLC Method for Determination of Modafinil in Human Plasma. Int. J. Nanomed. 2020, 15, 5253–5264. [Google Scholar] [CrossRef] [PubMed]
- Damle Mrinalini, C.; Deosthali Ajinkya, A. Development and Validation of HPLC Method for Determination of Modafinil in Human Plasma. Int. J. Pharm. Technol. 2016, 8, 11932–11942. [Google Scholar]
- Eissa, E.M.; Elkomy, M.H.; Eid, H.M.; Ali, A.A.; Abourehab, M.A.S.; Alsubaiyel, A.M.; Naguib, I.A.; Alsalahat, I.; Hassan, A.H. Intranasal Delivery of Granisetron to the Brain via Nanostructured Cubosomes-Based In Situ Gel for Improved Management of Chemotherapy-Induced Emesis. Pharmaceutics 2022, 14, 1374. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Chaudhary, S.; Shah, H.; Jacob, S.; Mewada, V.; Shinu, P.; Shah, J. Intranasal Delivery of Darunavir-Loaded Mucoadhesive In Situ Gel: Experimental Design, In Vitro Evaluation, and Pharmacokinetic Studies. Gels 2022, 8, 342. [Google Scholar] [CrossRef]






| Formulation Code | Gelation Temperature °C | Gelation Time (Seconds) |
|---|---|---|
| F1 | >36 | ND ** |
| F2 | >36 | ND ** |
| F3 | >36 | ND ** |
| F4 | >36 | ND ** |
| F5 | 35.5 | 55 |
| F6 | 34.9 | 49 |
| F7 | 36.3 | 52 |
| F8 | 34.6 | 48 |
| F9 | 32.4 | 25 |
| F10 | 33.3 | 35 |
| F11 | 29.5 | 15 |
| F12 | 28 | IN * |
| Formula | # Q4h (µg/cm2) | # Jss (µg/cm2·min) | # Peff (cm2/min) |
|---|---|---|---|
| ME-based nanogel | 603.55 ± 91 △ | 1.56 ± 0.9 △ | 0.156 ± 0.03 △ |
| Control gel | 420.3 ± 64 | 1.24 ± 0.4 | 0.124 ± 0.05 |
| Pharmacokinetic Parameters * | Propranolol Solution (Oral Route) | Propranolol Nanogel (Intranasal Route) |
|---|---|---|
| Cmax (ng/g) | 277.7 ± 29.71 | 970.3 ± 43.94 |
| AUC0–7 (ng·h/g) | 1518.35 ± 91.78 | 4884.15 ± 74.90 |
| AUC0–∞ (ng·h/g) | 1829.25 ± 98.01 | 6995.15 ± 77.39 |
| Ke (h−1) | 0.369521 ± 0.001 | 0.25775 ± 0.003 |
| Tmax (h) | 1.89 | 1.75 |
| t1/2 (h) | 1.87 ± 0.8 | 2.69 ± 1.02 |
| Relative availability (%) | - | 382.4 |
| Formulation | Poloxamer (%w/w) | Chitosan (%w/w) |
|---|---|---|
| F1 | 20 | 0.5 |
| F2 | 20 | 1 |
| F3 | 20 | 2 |
| F4 | 22 | 0.5 |
| F5 | 22 | 1 |
| F6 | 22 | 2 |
| F7 | 24 | 0.5 |
| F8 | 24 | 1 |
| F9 | 24 | 2 |
| F10 | 26 | 0.5 |
| F11 | 26 | 1 |
| F12 | 26 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abla, K.K.; Domiati, S.; El Majzoub, R.; Mehanna, M.M. Propranolol-Loaded Limonene-Based Microemulsion Thermo-Responsive Mucoadhesive Nasal Nanogel: Design, In Vitro Assessment, Ex Vivo Permeation, and Brain Biodistribution. Gels 2023, 9, 491. https://doi.org/10.3390/gels9060491
Abla KK, Domiati S, El Majzoub R, Mehanna MM. Propranolol-Loaded Limonene-Based Microemulsion Thermo-Responsive Mucoadhesive Nasal Nanogel: Design, In Vitro Assessment, Ex Vivo Permeation, and Brain Biodistribution. Gels. 2023; 9(6):491. https://doi.org/10.3390/gels9060491
Chicago/Turabian StyleAbla, Kawthar K., Souraya Domiati, Rania El Majzoub, and Mohammed M. Mehanna. 2023. "Propranolol-Loaded Limonene-Based Microemulsion Thermo-Responsive Mucoadhesive Nasal Nanogel: Design, In Vitro Assessment, Ex Vivo Permeation, and Brain Biodistribution" Gels 9, no. 6: 491. https://doi.org/10.3390/gels9060491