Theoretical and Experimental Aspects of Sodium Diclofenac Salt Release from Chitosan-Based Hydrogels and Possible Applications
Abstract
:1. Introduction
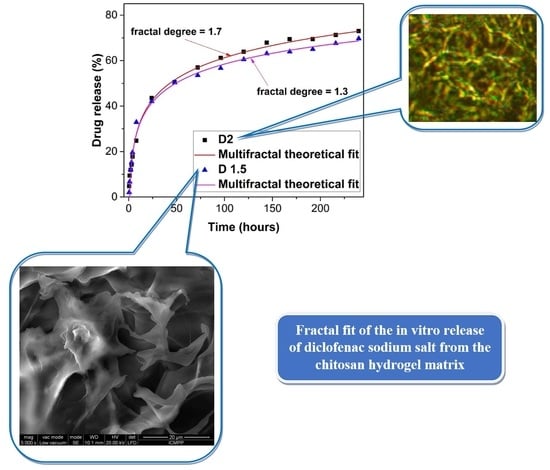
2. Results and Discussion
Correspondence of the Theoretical Model with the Experimental Data
3. Conclusions
4. Matherials and Methods
4.1. Generalities
4.2. Towards a Multifractal Pharmacokinetics
- (i)
- Monofractal dynamics, when the drug-release dynamics are characterized by a certain fractal dimension of the drug-release curves. In this way, for drug-release curves with (Peano-type curves [37,38]), Fickian-type drug-release regimes can be “mimed”, while for drug-release curves with , non-Fickian-type drug-release regimes can be “mimed”.
- (ii)
- Multifractal dynamics, when the drug-release dynamics are simultaneously characterized by more than one fractal dimension of the drug-release curves. In this way, mixed drug-release regimes (both of a Fickian and non-Fickian type) can be “mimed”. We note that it can be regularly found that for correlative drug-release modes and for non-correlative drug-release modes (for details on correlative and non-correlative-type behaviors see [37]).
4.3. Drug-Release Regimes through Harmonic Mappings
- (i)
- (ii)
- At any resolution scale, harmonic mappings from the usual space to the hyperbolic one by means of the stationary values of a Lagrangian connected to the metric Equation (10a).
- (iii)
- At any resolution scale, field equations (of a Euler–Lagrange-type) connected to the metric Equation (10a)
- (i)
- At any resolution scale, a transition from stationary to non-stationary states in the polymer–drug dynamics by choosing , in which case Equation (12) takes the form:
- (ii)
- At any resolution scale, the SL(2R) group, as an invariance group with respect to the metric Equation (10a), operates also as a synchronization group in drug-release dynamics.
4.4. Materials
4.5. Preparation of the Formulations
4.6. Characterization of the Hydrogel Formulations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Key Elements of Non-Differentiability Calibrated in Drug-Release Processes
Appendix B. Patterns and Drug-Release Mechanisms through Harmonic Mappings
Appendix C. Self-Similar Properties of the Polymer–Drug Dynamics at Differentiable and Non-Differentiable Scale Resolutions




References
- Vane, J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 1971, 231, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Grosser, T.; Fries, S.; FitzGerald, G.A. Biological basis for the cardiovascular consequences of COX-2 inhibition: Therapeutic challenges and opportunities. J. Clin. Investig. 2006, 116, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Leidgens, V.; Seliger, C.; Jachnik, B.; Welz, T.; Leukel, P.; Vollmann-Zwerenz, A.; Bogdahn, U.; Kreutz, M.; Grauer, O.M.; Hau, P. Ibuprofen and Diclofenac Restrict Migration and Proliferation of Human Glioma Cells by Distinct Molecular Mechanisms. PLoS ONE 2015, 10, e0140613. [Google Scholar] [CrossRef] [PubMed]
- Hamoya, T.; Fujii, G.; Miyamoto, S.; Takahashi, M.; Totsuka, Y.; Wakabayashi, K.; Toshima, J.; Mutoh, M. Effects of NSAIDs on the risk factors of colorectal cancer: A mini review. Genes Environ. Off. J. Jpn. Environ. Mutagen Soc. 2016, 38, 6. [Google Scholar] [CrossRef]
- Hofer, M.; Hoferova, Z.; Fedorocko, P.; Mackova, N.O. Hematopoiesis-stimulating and anti-tumor effects of repeated administration of diclofenac in mice with transplanted fibrosarcoma cells. Physiol. Res. 2002, 51, 629–632. [Google Scholar]
- Mayorek, N.; Naftali-Shani, N.; Grunewald, M. Diclofenac inhibits tumor growth in a murine model of pancreatic cancer by modulation of VEGF levels and arginase activity. PLoS ONE 2010, 5, e12715. [Google Scholar] [CrossRef]
- Connolly, T.P. Cyclooxigenase-2 inhibitors in gynecologic practice. Clin. Med. Res. 2003, 1, 105–110. [Google Scholar] [CrossRef]
- Chakraborty, I.; Das, S.K.; Wang, J.; Dey, S.K. Developmental expression of the cyclo-oxygenase-1 and cyclo-oxygenase-2 genes in the peri-implantation mouse uterus and their differential regulation by the blastocyst and ovarian steroids. J. Mol. Endocrinol. 1996, 16, 107–122. [Google Scholar] [CrossRef]
- Roongsitthichai, A.; Srisuwatanasagul, S.; Koonjaenak, S.; Tummaruk, P. Expression of cyclooxygenase-2 in the endometrium of gilts with different stages of endometritis. J. Vet. Med. Sci. 2011, 73, 1425–1431. [Google Scholar] [CrossRef]
- Altman, R.; Bosch, B.; Brune, K.; Patrignani, P.; Clarence, Y. Advances in NSAID development: Evolution of diclofenac products using pharmaceutical technology. Drugs 2015, 75, 859–877. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite nanotubes coated by chitosan for the controlled release of khellin. Polymers 2020, 12, 1766. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Micciulla, S.; Chiappisi, L.; Lazzara, G. Chitosan-based smart hybrid materials: A physico-chemical perspective. J. Mater. Chem. B 2021, 9, 594–611. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shangguan, Y.; Zheng, Q. Dynamics and Rheological Behavior of Chitosan-Grafted-Polyacrylamide in Aqueous Solution upon Heating. Polymers 2020, 12, 916. [Google Scholar] [CrossRef]
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Olaru, A.M.; Marin, L.; Morariu, S.; Pricope, G.; Pinteala, M.; Tartau-Mititelu, L. Biocompatible chitosan based hydrogels for potential application in local tumor therapy. Carbohydr. Polym. 2018, 179, 59–70. [Google Scholar] [CrossRef]
- Craciun, A.M.; Mititelu-Tartau, L.; Gavril, G.; Marin, L. Chitosan crosslinking with pyridoxal 5-phosphate vitamer toward biocompatible hydrogels for in vivo applications. Int. J. Biol. Macromol. 2022, 193, 1734–1743. [Google Scholar] [CrossRef]
- Marin, L.; Ailincai, D.; Morariu, S.; Tartau-Mititelu, L. Development of biocompatible glycodynameric hydrogels joining two natural motifs by dynamic constitutional chemistry. Carbohydr. Polym. 2017, 170, 60–71. [Google Scholar] [CrossRef]
- Craciun, A.M.; MititeluTartau, L.; Pinteala, M.; Marin, L. Nitrosalicyl-imine-chitosan hydrogels based drug delivery systems for long term sustained release in local therapy. J. Colloid Interface Sci. 2019, 536, 196–207. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campoes, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future perspectives. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Iftime, M.M.; Morariu, S.; Marin, L. Salicyl-imine-chitosan hydrogels: Supramolecular architecturing as a crosslinking method toward multifunctional hydrogels. Carbohydr. Polym. 2017, 165, 39–50. [Google Scholar] [CrossRef]
- Craciun, A.M.; Morariu, S.; Marin, L. Self-Healing Chitosan Hydrogels: Preparation and Rheological Characterization. Polymers 2022, 14, 2570. [Google Scholar] [CrossRef] [PubMed]
- Prokhorov, E.; Luna-Bárcenas, G.; Limón, J.M.Y.; Sánchez, A.G.; Kovalenko, Y. Chitosan-ZnO Nanocomposites Assessed by Dielectric, Mechanical, and Piezoelectric Properties. Polymers 2020, 12, 1991. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef]
- Bejan, A.; Doroftei, F.; Cheng, X.; Marin, L. Phenothiazine-chitosan based eco-adsorbents: A special design for mercury removal and fast naked eye detection. Int. J. Biol. Macromol. 2020, 162, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Fisher, O.Z.; Khademhosseini, A.; Pepas, N.A. Drug delivery: Nanoscale devices. In Encyclopedia of Materials: Science and Technology, 2nd ed.; Elsevier: New York, NY, USA, 2010; pp. 1–9. [Google Scholar]
- Kosmidis, K.; Ardyrakis, P.; Macheras, P. Fractal kinetics in drug release from finite matrices. J. Chem. Phys. 2003, 119, 6373–6377. [Google Scholar] [CrossRef]
- Marin, L.; Destri, S.; Porzio, W.; Bertini, F. Synthesis and characterization of new azomethine derivatives exhibiting liquid crystalline properties. Liq. Cryst. 2019, 36, 21–32. [Google Scholar] [CrossRef]
- Nikolova, D.; Simeonov, M.; Tzachev, C.; Apostolov, A.; Christov, L.; Vassileva, E. Polyelectrolyte complexes of chitosan and sodium alginate as a drug delivery system for diclofenac sodium. Polym. Int. 2022, 71, 668–678. [Google Scholar] [CrossRef]
- Chopra, L.; Thakur, K.K.; Chohan, J.S.; Sharma, S.; Ilyas, R.A.; Asyraf, M.R.M.; Zakaria, S.Z.S. Comparative Drug Release Investigations for Diclofenac Sodium Drug (DS) by Chitosan-Based Grafted and Crosslinked Copolymers. Materials 2022, 15, 2404. [Google Scholar] [CrossRef]
- Ghiorghita, C.A.; Dinu, M.V.; Dragan, E.S. Burst-free and sustained release of diclofenac sodium from mesoporous silica/PEI microspheres coated with carboxymethyl cellulose/chitosan layer-by-layer films. Cellulose 2022, 29, 395–412. [Google Scholar] [CrossRef]
- Andreica, B.I.; Ailincai, D.; Sandu, A.I.; Marin, L. Amphiphilic chitosan-g-poly(trimethylene carbonate)-A new approach for biomaterials design. Int. J. Biol. Macromol. 2021, 193, 414–424. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, L.; Wang, L.; Gao, Y.; Cui, W.; Chu, D.; Zhang, Y. Chitosan combined with intrauterine device prevents intrauterine adhesions after hysteroscopic adhesiolysis: A target trial emulation study. J Obstet. Gynaecol. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liang, L. Thermal sensitive Poloxamer/Chitosan hydrogel for drug delivery in vagina. Mater. Res. Express 2020, 7, 105401. [Google Scholar] [CrossRef]
- Araujo, V.H.S.; de Souza, M.P.C.; Carvalho, G.C.; Duarte, J.L.; Chorilli, M. Chitosan-based systems aimed at local application for vaginal infections. Carbohydr. Polym. 2021, 261, 117919. [Google Scholar] [CrossRef]
- Babu, A.; Ramesh, R. Multifaceted Applications of Chitosan in Cancer Drug Delivery and Therapy. Mar. Drugs 2017, 15, 96. [Google Scholar] [CrossRef]
- Toma, B.F.; Socolov, R.; Popa, O.; Socolov, D.; Nica, I.; Agop, M.; Vasincu, D.; Grigore, M.; Ochiuz, L. Prospects and challenges of the drug delivery systems in endometriosis pain management: Experimental and theoretical aspects. J. Immunol. Res. 2021, 2021, 2727174. [Google Scholar] [CrossRef]
- Mandlebrot, B.B. The Fractal Geometry of Nature; W.H. Freeman and Co.: San Francisco, CA, USA, 1982. [Google Scholar]
- Nottale, L. Scale Relativity and Fractal Space-Time: A New Approach to Unifying Relativity and Quantum Mechanics; World Scientific Publishing Co., Pte. Ltd.: London, UK, 2011. [Google Scholar]
- Merches, I.; Agop, M. Differentiability and Fractality in Dynamics of Physical Systems; World Scientific: Hackensack, NJ, USA, 2016. [Google Scholar]
- Agop, M.; Paun, V.P. On the New Perspectives of Fractal Theory. Fundaments and Applications; Romanian Academy Publishing House: Bucharest, Romania, 2017. [Google Scholar]
- Iftime, M.M.; Dobreci, D.L.; Irimiciuc, S.A.; Agop, M.; Petrescu, T.; Doroftei, B. A theoretical mathematical model for assessing diclofenac release from chitosan-based formulations. Drug Deliv. 2020, 27, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Marin, L.; Popescu, M.C.; Zabulica, A.; Uji-I, H.; Fron, E. Chitosan as a matrix for biopolymer dispersed liquid crystal systems. Carbohydr. Polym. 2013, 95, 16–24. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Himiniuc, L.M.; Socolov, R.; Nica, I.; Agop, M.; Volovat, C.; Ochiuz, L.; Vasincu, D.; Rotundu, A.M.; Rosu, I.A.; Ghizdovat, V.; et al. Theoretical and Experimental Aspects of Sodium Diclofenac Salt Release from Chitosan-Based Hydrogels and Possible Applications. Gels 2023, 9, 422. https://doi.org/10.3390/gels9050422
Himiniuc LM, Socolov R, Nica I, Agop M, Volovat C, Ochiuz L, Vasincu D, Rotundu AM, Rosu IA, Ghizdovat V, et al. Theoretical and Experimental Aspects of Sodium Diclofenac Salt Release from Chitosan-Based Hydrogels and Possible Applications. Gels. 2023; 9(5):422. https://doi.org/10.3390/gels9050422
Chicago/Turabian StyleHiminiuc, Loredana Maria, Razvan Socolov, Irina Nica, Maricel Agop, Constantin Volovat, Lacramioara Ochiuz, Decebal Vasincu, Ana Maria Rotundu, Iulian Alin Rosu, Vlad Ghizdovat, and et al. 2023. "Theoretical and Experimental Aspects of Sodium Diclofenac Salt Release from Chitosan-Based Hydrogels and Possible Applications" Gels 9, no. 5: 422. https://doi.org/10.3390/gels9050422