Injectable Thermoresponsive Hydrogels for Cancer Therapy: Challenges and Prospects
Abstract
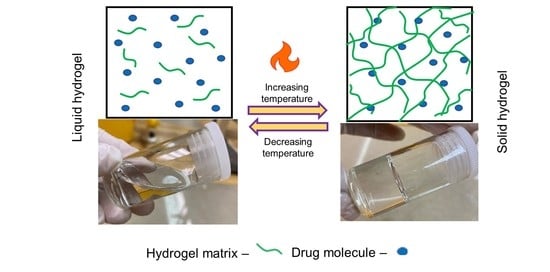
:1. Introduction
2. Physiological Barriers to Drug Delivery in Cancerous Tumours
3. Selection and Preparation of Injectable Thermosensitive Hydrogels
3.1. Physical vs. Chemical Crosslinking
3.2. Natural vs. Synthetic Hydrogels
3.3. The Drug-Loading Dilemma
| Drug | Type of Cancer Commonly Indicated for | Solubility in Aqueous Solution (mg/mL) | Examples of Thermoresponsive Delivery Systems | Reference |
|---|---|---|---|---|
| Cisplatin | Prostate, ovarian, and bladder cancer | ~1 | Co-delivery of resveratrol microspheres and cisplatin into pluronic-F127 hydrogel against H22 cells. | [54,55] |
| Paclitaxel | Breast, colon, and recurrent ovarian cancer | ~0.002 | Paclitaxel nanocrystals loaded into poloxamer 407, poloxamer 188, and carbomer 974P against breast cancer. | [52,56,57] |
| Doxorubicin | Leukemia, breast cancer, soft tissue and bone sarcoma, ovarian, bladder, thyroid, and gastric carcinoma | <10 | Co-delivery of doxorubicin and cisplatin loaded in PLGA-PEG-PLGA hydrogel against Saos-2 and MG-63 cells. | [58,59] |
| Docetaxel | Prostate cancer, metastatic breast cancer, and gastric cancer | 0.006–0.007 | Black phosphorus nanosheets and micelle docetaxel loaded in PF-127 thermoreversible hydrogel for chemo-photodynamic therapy. | [60,61] |
| Daunorubicin | Leukemia | ~0.3 | - | [62] |
| Tamoxifen | Breast Cancer | ~0.0003 | Tamoxifen nanoparticles loaded in PLGA-PEG-PLGA against MCF-7 cells in breast cancer. | [63,64] |
| Etoposide | Testicular, prostate, bladder, stomach, and lung cancer | ∼0.008 | Etoposide loaded in poloxamer 407/poloxamer 188 thermosensitive hydrogel for sustained drug release. | [65,66,67] |
| Irinotecan | Colorectal cancer | ~0.107 | Irinotecan-loaded solid lipid nanoparticles in a poloxamer 407/polaxamer 188 thermosensitive hydrogel for colorectal cancer. | [68] |
| 5-fluorouracil | Breast, colorectal, stomach, and pancreatic cancer | 12 | 5-fluorouracil loaded into polaxamer 407/polaxamer 188/alginate thermosensitive hydrogel for colorectal cancer. | [69] |
| Methotrexate | Non-Hodgkin’s lymphoma, breast, ovarian, and lung cancer, and epidermal tumors of the head and neck. | ~0.067 | Methotrexate carbon nanotubes were loaded into a chitosan/β-glycerophosphate thermosensitive hydrogel. | [70] |
| Bleomycin | Squamous cell carcinoma of the head and neck, testicular carcinoma, Hodgkin lymphoma | 20 | Bleomycin liposomes loaded in PF-127/PF-68 thermosensitive hydrogel. | [71] |
3.4. Lower Critical Solution Temperature
3.5. Dynamics of Drug Release
3.6. Physical Mixing Hurdles
4. Administration of Injectable Thermosensitive Hydrogels
5. Thermoresponsive Hydrogels in Clinical Trials: An Update
| Trade Name | Encapsulated Drug | Thermosensitive Hydrogel | Cancer Type | Status | References |
|---|---|---|---|---|---|
| OncoGel® | Paclitaxel | PLGA-PEG-PLGA | Esophageal cancer; adenocarcinoma of the esophagus; squamous cell carcinoma; brain neoplasms; | Phase 2 | [126,128] |
| glioblastoma multiforme | Phase 2 | [131] | |||
| Jelymyto® | Mytomycin | PLGA-PEG-PLGA | Carcinoma; transitional cell; transitional cell; carcinoma of renal pelvis; | Phase 3 | [131,133] |
| bladder cancer | Phase 2 | [136] |
6. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chatterjee, S.; Chi-leung Hui, P.; Kan, C. Thermoresponsive Hydrogels and Their Biomedical Applications: Special Insight into Their Applications in Textile Based Transdermal Therapy. Polymers 2018, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, Z.; Liu, J.; Li, H.; Su, Q.; Zhang, J.; Huang, P.; Wang, W.; Liu, J. Polarization of tumor-associated macrophages by TLR7/8 conjugated radiosensitive peptide hydrogel for overcoming tumor radioresistance. Bioact. Mater. 2022, 16, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, W.; Qian, J.; Wang, Y.; Hou, G.; Suo, A. Photo-crosslinked hyaluronic acid hydrogel as a biomimic extracellular matrix to recapitulate in vivo features of breast cancer cells. Colloids Surf. B Biointerfaces 2022, 209, 112159. [Google Scholar] [CrossRef]
- García-Couce, J.; Tomás, M.; Fuentes, G.; Que, I.; Almirall, A.; Cruz, L.J. Chitosan/Pluronic F127 Thermosensitive Hydrogel as an Injectable Dexamethasone Delivery Carrier. Gels 2022, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Song, X.; Wen, Y.; Zhu, J.-L.; Li, J. Injectable Thermoresponsive Hydrogel Formed by Alginate-g-Poly(N-isopropylacrylamide) That Releases Doxorubicin-Encapsulated Micelles as a Smart Drug Delivery System. ACS Appl. Mater. Interfaces 2017, 9, 35673–35682. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, D.; He, C.; Sun, W.; Cao, R.; Cui, S.; Deng, M.; Gu, Z.; Chen, X. Injectable Thermosensitive Polypeptide-Based CDDP-Complexed Hydrogel for Improving Localized Antitumor Efficacy. Biomacromolecules 2017, 18, 4341–4348. [Google Scholar] [CrossRef]
- Darge, H.F.; Andrgie, A.T.; Hanurry, E.Y.; Birhan, Y.S.; Mekonnen, T.W.; Chou, H.-Y.; Hsu, W.-H.; Lai, J.-Y.; Lin, S.-Y.; Tsai, H.-C. Localized controlled release of bevacizumab and doxorubicin by thermo-sensitive hydrogel for normalization of tumor vasculature and to enhance the efficacy of chemotherapy. Int. J. Pharm. 2019, 572, 118799. [Google Scholar] [CrossRef]
- Babaei, M.; Davoodi, J.; Dehghan, R.; Zahiri, M.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Thermosensitive composite hydrogel incorporated with curcumin-loaded nanopolymersomes for prolonged and localized treatment of glioma. J. Drug Deliv. Sci. Technol. 2020, 59, 101885. [Google Scholar] [CrossRef]
- Li, R.; Lin, Z.; Zhang, Q.; Zhang, Y.; Liu, Y.; Lyu, Y.; Li, X.; Zhou, C.; Wu, G.; Ao, N.; et al. Injectable and In Situ-Formable Thiolated Chitosan-Coated Liposomal Hydrogels as Curcumin Carriers for Prevention of In Vivo Breast Cancer Recurrence. ACS Appl. Mater. Interfaces 2020, 12, 17936–17948. [Google Scholar] [CrossRef]
- Bai, R.; Deng, X.; Wu, Q.; Cao, X.; Ye, T.; Wang, S. Liposome-loaded thermo-sensitive hydrogel for stabilization of SN-38 via intratumoral injection: Optimization, characterization, and antitumor activity. Pharm. Dev. Technol. 2018, 23, 106–115. [Google Scholar] [CrossRef]
- Han, X.; Meng, X.; Wu, Z.; Wu, Z.; Qi, X. Dynamic imine bond cross-linked self-healing thermosensitive hydrogels for sustained anticancer therapy via intratumoral injection. Mater. Sci. Eng. C 2018, 93, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Sheu, M.T.; Jhan, H.J.; Su, C.Y.; Chen, L.C.; Chang, C.E.; Liu, D.Z.; Ho, H.O. Codelivery of doxorubicin-containing thermosensitive hydrogels incorporated with docetaxel-loaded mixed micelles enhances local cancer therapy. Colloids Surf. B Biointerfaces 2016, 143, 260–270. [Google Scholar] [CrossRef]
- Petrova, V.; Annicchiarico-Petruzzelli, M.; Melino, G.; Amelio, I. The hypoxic tumour microenvironment Hypoxia and hypoxia-inducible factors. Oncogenesis 2018, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shi, X.; Wu, D.; Kahsay Khshen, F.; Deng, L.; Dong, A.; Wang, W.; Zhang, J. Injectable, Biodegradable, Thermosensitive Nanoparticles-Aggregated Hydrogel with Tumor-Specific Targeting, Penetration, and Release for Efficient Postsurgical Prevention of Tumor Recurrence. ACS Appl. Mater. Interfaces 2019, 11, 19700–19711. [Google Scholar] [CrossRef]
- Shi, X.; Wu, J.; Wang, Z.; Song, F.; Gao, W.; Liu, S. Synthesis and properties of a temperature-sensitive hydrogel based on physical crosslinking via stereocomplexation of PLLA-PDLA. RSC Adv. 2020, 10, 19759–19769. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleki, A.; Gharibi, H.; Molavian, M.R.; Norouzi, M.; Asefifeyzabadi, N. Physicochemical modification of hydroxylated polymers to develop thermosensitive double network hydrogels. J. Appl. Polym. Sci. 2021, 138, 50778. [Google Scholar] [CrossRef]
- Choi, J.; Yoon, J.; Ahn, K.H.; Choi, S.-H.; Char, K. Injectable hydrogels with improved mechanical property based on electrostatic associations. Colloid Polym. Sci. 2021, 299, 575–584. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Doroudian, M.; Ahadpour, A.; Azari, S. Injectable chitosan/κ-carrageenan hydrogel designed with au nanoparticles: A conductive scaffold for tissue engineering demands. Int. J. Biol. Macromol. 2019, 126, 310–317. [Google Scholar] [CrossRef]
- Yeh, M.-Y.; Zhao, J.-Y.; Hsieh, Y.-R.; Lin, J.-H.; Chen, F.-Y.; Chakravarthy, R.D.; Chung, P.-C.; Lin, H.-C.; Hung, S.-C. Reverse thermo-responsive hydrogels prepared from Pluronic F127 and gelatin composite materials. RSC Adv. 2017, 7, 21252–21257. [Google Scholar] [CrossRef]
- Dehghan-Baniani, D.; Chen, Y.; Wang, D.; Bagheri, R.; Solouk, A.; Wu, H. Injectable in situ forming kartogenin-loaded chitosan hydrogel with tunable rheological properties for cartilage tissue engineering. Colloids Surf. B Biointerfaces 2020, 192, 111059. [Google Scholar] [CrossRef]
- Maiti, B.; Abramov, A.; Franco, L.; Puiggalí, J.; Enshaei, H.; Alemán, C.; Díaz Díaz, D.; Maiti, B.; Abramov, A.; Díaz, D.D.; et al. Thermoresponsive Shape-Memory Hydrogel Actuators Made by Phototriggered Click Chemistry. Adv. Funct. Mater. 2020, 30, 2001683. [Google Scholar] [CrossRef]
- Liao, S.; Tang, L.; Qu, J. Schiff-base-functionalized polymeric hydrogel with high stretchability and multifunction. Polym. Adv. Technol. 2021, 32, 1844–1852. [Google Scholar] [CrossRef]
- Wu, S.W.; Liu, X.; Miller, A.L.; Cheng, Y.S.; Yeh, M.L.; Lu, L. Strengthening injectable thermo-sensitive NIPAAm-g-chitosan hydrogels using chemical cross-linking of disulfide bonds as scaffolds for tissue engineering. Carbohydr. Polym. 2018, 192, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Panyamao, P.; Ruksiriwanich, W.; Sirisa-Ard, P.; Charumanee, S. Injectable Thermosensitive Chitosan/Pullulan-Based Hydrogels with Improved Mechanical Properties and Swelling Capacity. Polymers 2020, 12, 2514. [Google Scholar] [CrossRef]
- Drozdov, A.D.; de Claville Christiansen, J. Modulation of the volume phase transition temperature for multi-stimuli-responsive copolymer hydrogels. Int. J. Mech. Sci. 2021, 211, 106753. [Google Scholar] [CrossRef]
- Ramírez Barragán, C.A.; Macías Balleza, E.R.; García-Uriostegui, L.; Andrade Ortega, J.A.; Toríz, G.; Delgado, E. Rheological characterization of new thermosensitive hydrogels formed by chitosan, glycerophosphate, and phosphorylated β-cyclodextrin. Carbohydr. Polym. 2018, 201, 471–481. [Google Scholar] [CrossRef]
- Qin, H.; Wang, J.; Wang, T.; Gao, X.; Wan, Q.; Pei, X. Preparation and Characterization of Chitosan/β-Glycerophosphate Thermal-Sensitive Hydrogel Reinforced by Graphene Oxide. Front. Chem. 2018, 6, 565. [Google Scholar] [CrossRef]
- Abrami, M.; Siviello, C.; Grassi, G.; Larobina, D.; Grassi, M. Investigation on the thermal gelation of Chitosan/β-Glycerophosphate solutions. Carbohydr. Polym. 2019, 214, 110–116. [Google Scholar] [CrossRef]
- Deng, A.; Kang, X.; Zhang, J.; Yang, Y.; Yang, S. Enhanced gelation of chitosan/β-sodium glycerophosphate thermosensitive hydrogel with sodium bicarbonate and biocompatibility evaluated. Mater. Sci. Eng. C 2017, 78, 1147–1154. [Google Scholar] [CrossRef]
- Al-Sibani, M.; Al-Harrasi, A.; Neubert, R.H.H. Evaluation of in-vitro degradation rate of hyaluronic acid-based hydrogel cross-linked with 1,4-butanediol diglycidyl ether (BDDE) using RP-HPLC and UV–Vis spectroscopy. J. Drug Deliv. Sci. Technol. 2015, 29, 24–30. [Google Scholar] [CrossRef]
- Lu, S.; Yang, Y.; Yao, J.; Shao, Z.; Chen, X. Exploration of the nature of a unique natural polymer-based thermosensitive hydrogel. Soft Matter 2016, 12, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Jhan, H.-J.; Liu, J.-J.; Chen, Y.-C.; Liu, D.-Z.; Sheu, M.-T.; Ho, H.-O. Novel injectable thermosensitive hydrogels for delivering hyaluronic acid–doxorubicin nanocomplexes to locally treat tumors. Nanomedicine 2015, 10, 1263–1274. [Google Scholar] [CrossRef]
- Kim, J.K.; Won, Y.-W.; Suk Lim, K.; Kim, Y.-H. Low-Molecular-Weight Methylcellulose-Based Thermo-reversible Gel/Pluronic Micelle Combination System for Local and Sustained Docetaxel Delivery. Pharm. Res. 2011, 29, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Liu, R.; Hu, L.-Q.; Wang, J.-H.; Si, C.-L. Self-assembled PEG-carboxymethylcellulose nanoparticles/a-cyclodextrin hydrogels for injectable and thermosensitive drug delivery. RSC Adv. 2017, 7, 2905–2912. [Google Scholar] [CrossRef]
- Zhang, D.; Chu, Y.; Qian, H.; Qian, L.; Shao, J.; Xu, Q.; Yu, L.; Li, R.; Zhang, Q.; Wu, F.; et al. Antitumor Activity of Thermosensitive Hydrogels Packaging Gambogic Acid Nanoparticles and Tumor-Penetrating Peptide iRGD Against Gastric Cancer. Int. J. Nanomed. 2020, 15, 735–747. [Google Scholar] [CrossRef]
- Chen, H.; Fan, M. Novel Thermally Sensitive pH-dependent Chitosan/Carboxymethyl Cellulose Hydrogels. J. Bioact. Compat. Polym. 2008, 23, 38–48. [Google Scholar] [CrossRef]
- Mellati, A.; Valizadeh Kiamahalleh, M.; Dai, S.; Bi, J.; Jin, B.; Zhang, H. Influence of polymer molecular weight on the in vitro cytotoxicity of poly (N-isopropylacrylamide). Mater. Sci. Eng. C 2016, 59, 509–513. [Google Scholar] [CrossRef]
- Patenaude, M.; Hoare, T. Injectable, Degradable Thermoresponsive Poly(N-isopropylacrylamide) Hydrogels. ACS Macro Lett. 2012, 1, 409–413. [Google Scholar] [CrossRef]
- Rezazadeh, M.; Akbari, V.; Amuaghae, E.; Emami, J. Preparation and characterization of an injectable thermosensitive hydrogel for simultaneous delivery of paclitaxel and doxorubicin. Res. Pharm. Sci. 2018, 13, 181. [Google Scholar]
- Jin, X.; Fu, Q.; Gu, Z.; Zhang, Z.; Lv, H. Injectable corilagin/low molecular weight chitosan/PLGA-PEG-PLGA thermosensitive hydrogels for localized cancer therapy and promoting drug infiltration by modulation of tumor microenvironment. Int. J. Pharm. 2020, 589, 119772. [Google Scholar] [CrossRef]
- Zhao, D.; Song, H.; Zhou, X.; Chen, Y.; Liu, Q.; Gao, X.; Zhu, X.; Chen, D. Novel facile thermosensitive hydrogel as sustained and controllable gene release vehicle for breast cancer treatment. Eur. J. Pharm. Sci. 2019, 134, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, H.; Jia, Y.; Guo, Q.; Qu, Y.; Su, J.; Lu, X.; Zhao, Y.; Qian, Z. A novel gene delivery composite system based on biodegradable folate-poly (ester amine) polymer and thermosensitive hydrogel for sustained gene release. Sci. Rep. 2016, 6, 21402. [Google Scholar] [CrossRef] [PubMed]
- Chaibundit, C.; Ricardo, N.M.P.S.; Ricardo, N.M.P.S.; Costa, F.D.M.L.L.; Wong, M.G.P.; Hermida-Merino, D.; Rodriguez-Perez, J.; Hamley, I.W.; Yeates, S.G.; Booth, C. Effect of Ethanol on the Micellization and Gelation of Pluronic P123. Langmuir 2008, 24, 12260–12266. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Bae, Y.C. Thermodynamic framework for switching the lower critical solution temperature of thermo-sensitive particle gels in aqueous solvent. Polymer 2020, 195, 122428. [Google Scholar] [CrossRef]
- Xu, S.; Du, X.; Feng, G.; Zhang, Y.; Li, J.; Lin, B.; Yang, L.; Fu, S.; Wu, J. Efficient inhibition of cervical cancer by dual drugs loaded in biodegradable thermosensitive hydrogel composites. Oncotarget 2018, 9, 282. [Google Scholar] [CrossRef]
- Boonlai, W.; Tantishaiyakul, V.; Hirun, N.; Sangfai, T.; Suknuntha, K. Thermosensitive Poloxamer 407/Poly(Acrylic Acid) Hydrogels with Potential Application as Injectable Drug Delivery System. AAPS PharmSciTech 2018, 19, 2103–2117. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Castillo, T.D.; Luna-Velasco, A.; Zaragoza-Contreras, E.A.; Castro-Carmona, J.S. Thermosensitive hydrogel for in situ-controlled methotrexate delivery. e-Polymers 2021, 21, 910–920. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, J.; Lu, Y. Doxorubicin and CD-CUR inclusion complex co-loaded in thermosensitive hydrogel PLGA-PEG-PLGA localized administration for osteosarcoma. Int. J. Oncol. 2020, 57, 433–444. [Google Scholar] [CrossRef]
- Xu, S.; Wang, W.; Li, X.; Liu, J.; Dong, A.; Deng, L. Sustained release of PTX-incorporated nanoparticles synergized by burst release of DOX⋅HCl from thermosensitive modified PEG/PCL hydrogel to improve anti-tumor efficiency. Eur. J. Pharm. Sci. 2014, 62, 267–273. [Google Scholar] [CrossRef]
- Mao, Y.; Li, X.; Chen, G.; Wang, S. Thermosensitive Hydrogel System with Paclitaxel Liposomes Used in Localized Drug Delivery System for In Situ Treatment of Tumor: Better Antitumor Efficacy and Lower Toxicity. J. Pharm. Sci. 2016, 105, 194–204. [Google Scholar] [CrossRef]
- Zhou, X.; He, X.; Shi, K.; Yuan, L.; Yang, Y.; Liu, Q.; Ming, Y.; Yi, C.; Qian, Z. Injectable Thermosensitive Hydrogel Containing Erlotinib-Loaded Hollow Mesoporous Silica Nanoparticles as a Localized Drug Delivery System for NSCLC Therapy. Adv. Sci. 2020, 7, 2001442. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Sun, W.; Zhang, T.; Wang, R.; Tian, Y.; Zhang, H.; Li, J.; Zheng, A.; Song, S. Paclitaxel-nanocrystals-loaded network thermosensitive hydrogel for localised postsurgical recurrent of breast cancer after surgical resection. Biomed. Pharmacother. 2022, 150, 113017. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Hu, C.; Fu, Q.; Lv, H. Combined chemotherapy for triple negative breast cancer treatment by paclitaxel and niclosamide nanocrystals loaded thermosensitive hydrogel. Eur. J. Pharm. Sci. 2021, 167, 105992. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; De Dille, A.; Jameson, V.J.; Smith, L.; Dernell, W.S.; Manning, M.C. Improved potency of cisplatin by hydrophobic ion pairing. Cancer Chemother. Pharmacol. 2004, 54, 441–448. [Google Scholar] [CrossRef]
- Wen, Q.; Zhang, Y.; Luo, J.; Xiong, K.; Lu, Y.; Wu, Z.X.; Wang, B.Q.; Wu, J.B.; Chen, Y.; Fu, S.Z. Therapeutic efficacy of thermosensitive Pluronic hydrogel for codelivery of resveratrol microspheres and cisplatin in the treatment of liver cancer ascites. Int. J. Pharm. 2020, 582, 119334. [Google Scholar] [CrossRef] [PubMed]
- Dordunoo, S.K.; Burt, H.M. Solubility and stability of taxol: Effects of buffers and cyclodextrins. Int. J. Pharm. 1996, 133, 191–201. [Google Scholar] [CrossRef]
- Miwa, A.; Ishibe, A.; Nakano, M.; Yamahira, T.; Itai, S.; Jinno, S.; Kawahara, H. Development of novel chitosan derivatives as micellar carriers of taxol. Pharm. Res. 1998, 15, 1844–1850. [Google Scholar] [CrossRef]
- Si, M.; Xia, Y.; Cong, M.; Wang, D.; Hou, Y.; Ma, H. In situ Co-Delivery of Doxorubicin and Cisplatin by Injectable Thermosensitive Hydrogels for Enhanced Osteosarcoma Treatment. Int. J. Nanomed. 2022, 17, 1309. [Google Scholar] [CrossRef]
- Cell Signaling Technology: Doxorubicin. Available online: https://media.cellsignal.com/pdf/5927.pdf#:~:text=Solubility%3ASoluble%20in%20DMSO%20at%20100%20mg%2Fml%3B%20very%20poorly,Use%3A%20Doxorubicin%20is%20supplied%20as%20a%20lyophilized%20powder (accessed on 20 June 2022).
- Du, W.; Hong, L.; Yao, T.; Yang, X.; He, Q.; Yang, B.; Hu, Y. Synthesis and evaluation of water-soluble docetaxel prodrugs-docetaxel esters of malic acid. Bioorg. Med. Chem. 2007, 15, 6323–6330. [Google Scholar] [CrossRef]
- Li, R.; Shan, L.; Yao, Y.; Peng, F.; Jiang, S.; Yang, D.; Ling, G.; Zhang, P. Black phosphorus nanosheets and docetaxel micelles co-incorporated thermoreversible hydrogel for combination chemo-photodynamic therapy. Drug Deliv. Transl. Res. 2021, 11, 1133–1143. [Google Scholar] [CrossRef]
- Chu, L.L.; Pandey, R.P.; Shin, J.Y.; Jung, H.J.; Sohng, J.K. Synthetic analog of anticancer drug daunorubicin from daunorubicinone using one-pot enzymatic UDP-recycling glycosylation. J. Mol. Catal. B Enzym. 2016, 124, 1–10. [Google Scholar] [CrossRef]
- Öztürk-Atar, K.; Kaplan, M.; Çalış, S. Development and evaluation of polymeric micelle containing tablet formulation for poorly water-soluble drug: Tamoxifen citrate. Drug Dev. Ind. Pharm. 2020, 46, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Lei, H.; Zheng, X.; Han, Y.; Sun, R.; Zhao, D.; Liu, R. A temperature-sensitive phase-change hydrogel of tamoxifen achieves the long-acting antitumor activation on breast cancer cells. Onco Targets Ther. 2019, 12, 3919. [Google Scholar] [CrossRef]
- Soni, G.; Yadav, K.S. High encapsulation efficiency of poloxamer-based injectable thermoresponsive hydrogels of etoposide. Pharm. Dev. Technol. 2014, 19, 651–661. [Google Scholar] [CrossRef]
- Alsalhi, A.; Ayon, N.J.; Coulibaly, F.; Alshamrani, M.; Al-Nafisah, A.; Youan, B.-B.C. Enhancing Etoposide Aqueous Solubility and Anticancer Activity with L-Arginine. Assay Drug Dev. Technol. 2021, 19, 508–525. [Google Scholar] [CrossRef] [PubMed]
- Reyhanoglu, G.; Tadi, P. Etoposide. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557864/ (accessed on 20 June 2022).
- Ud Din, F.; Jin, S.G.; Choi, H.-G. Particle and Gel Characterization of Irinotecan-Loaded Double-Reverse Thermosensitive Hydrogel. Polymers 2021, 13, 551. [Google Scholar] [CrossRef]
- Al Sabbagh, C.; Seguin, J.; Agapova, E.; Kramerich, D.; Boudy, V.; Mignet, N. Thermosensitive hydrogels for local delivery of 5-fluorouracil as neoadjuvant or adjuvant therapy in colorectal cancer. Eur. J. Pharm. Biopharm. 2020, 157, 154–164. [Google Scholar] [CrossRef]
- Saeednia, L.; Yao, L.; Berndt, M.; Cluff, K.; Asmatulu, R. Structural and biological properties of thermosensitive chitosan-graphene hybrid hydrogels for sustained drug delivery applications. J. Biomed. Mater. Res. Part A 2017, 105, 2381–2390. [Google Scholar] [CrossRef]
- Zhang, N.; Zheng, S.; Pan, Z.; Liu, Z. Phase Transition Effects on Mechanical Properties of NIPA Hydrogel. Polymers 2018, 10, 358. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, Z.; Liu, Y.; Huang, Z.; Chai, C.; Hao, J. Multiple Cross-Linking-Dominated Metal−Ligand Coordinated Hydrogels with Tunable Strength and Thermosensitivity. ACS Appl. Polym. Mater. 2019, 1, 2370–2378. [Google Scholar] [CrossRef]
- Zeinali, E.; Haddadi-Asl, V.; Roghani-Mamaqani, H. Synthesis of dual thermo- and pH-sensitive poly(N-isopropylacrylamide-co-acrylic acid)-grafted cellulose nanocrystals by reversible addition-fragmentation chain transfer polymerization. J. Biomed. Mater. Res. A 2018, 106, 231–243. [Google Scholar] [CrossRef]
- Higashi, N.; Matsubara, S.; Nishimura, S.; Koga, T. Stepwise Thermo-Responsive Amino Acid-Derived Triblock Vinyl Polymers: ATRP Synthesis of Polymers, Aggregation, and Gelation Properties via Flower-like Micelle Formation. Materials 2018, 11, 424. [Google Scholar] [CrossRef]
- Razmimanesh, F.; Sodeifian, G. Investigation of Temperature-Responsive Tocosomal Nanocarriers as the Efficient and Robust Drug Delivery System for Sunitinib Malate Anti-Cancer Drug: Effects of MW and Chain Length of PNIPAAm on LCST and Dissolution Rate. J. Pharm. Sci. 2022, 111, 1937–1951. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Minhas, M.U.; Ahmad, M.; Sohail, M. Self-assembled supramolecular thermoreversible β-cyclodextrin/ethylene glycol injectable hydrogels with difunctional Pluronic® 127 as controlled delivery depot of curcumin. Development, characterization and in vitro evaluation. J. Biomater. Sci. 2018, 29, 1–34. [Google Scholar] [CrossRef]
- Safakas, K.; Saravanou, S.-F.; Iatridi, Z.; Tsitsilianis, C. Alginate-g-PNIPAM-Based Thermo/Shear-Responsive Injectable Hydrogels: Tailoring the Rheological Properties by Adjusting the LCST of the Grafting Chains. Int. J. Mol. Sci. 2021, 22, 3824. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Chen, J.P.; Leu, Y.L.; Hu, J.W. Temperature-sensitive hydrogels composed of chitosan and hyaluronic acid as injectable carriers for drug delivery. Eur. J. Pharm. Biopharm. 2008, 68, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Fehér, B.; Varga, I.; Pedersen, J.S. Effect of concentration and ionic strength on the lower critical solution temperature of poly(N-isopropylacrylamide) investigated by small-angle X-ray scattering. Soft Mater. 2021, 20, S10–S18. [Google Scholar] [CrossRef]
- Starovoytova, L.; Št’astná, J.; Šturcová, A.; Konefal, R.; Dybal, J.; Velychkivska, N.; Radecki, M.; Hanyková, L. Additive Effects on Phase Transition and Interactions in Poly(vinyl methyl ether) Solutions. Polymers 2015, 7, 2572–2583. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, H.; Ding, J. A subtle end-group effect on macroscopic physical gelation of triblock copolymer aqueous solutions. Angew. Chem. Int. Ed. 2006, 45, 2232–2235. [Google Scholar] [CrossRef]
- Marsili, L.; Dal Bo, M.; Eisele, G.; Donati, I.; Berti, F.; Toffoli, G.; Stadler, F.J. Characterization of Thermoresponsive Poly-N-Vinylcaprolactam Polymers for Biological Applications. Polymers 2021, 13, 2639. [Google Scholar] [CrossRef]
- Ahmadi, R.; de Bruijn, J.D. Biocompatibility and gelation of chitosan–glycerol phosphate hydrogels. J. Biomed. Mater. Res. Part A 2007, 86A, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.C. Sol-Gel Behavior of Hydroxypropyl Methylcellulose (HPMC) in Ionic Media Including Drug Release. Materials 2011, 4, 1861–1905. [Google Scholar] [CrossRef]
- Wilfert, S.; Iturmendi, A.; Henke, H.; Brüggemann, O.; Teasdale, I. Thermoresponsive Polyphosphazene-Based Molecular Brushes by Living Cationic Polymerization. Macromol. Symp. 2014, 337, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Tsai, C.; Lin, Y.; Chu, M. Study of novel biodegradable thermo-sensitive hydrogels of methoxy-poly(ethylene glycol)-block-polyester diblock copolymers. Polym. Int. 2010, 55, 1428–1435. [Google Scholar] [CrossRef]
- Huang, H.; Qi, X.; Chen, Y.; Wu, Z. Thermo-sensitive hydrogels for delivering biotherapeutic molecules: A review. Saudi Pharm. J. 2019, 27, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, C.; Huang, X.; Lin, X.; Lin, W.; Yang, F.; Chen, T. Thermosensitive hydrogels for sustained-release of sorafenib and selenium nanoparticles for localized synergistic chemoradiotherapy. Biomaterials 2019, 216, 119220. [Google Scholar] [CrossRef]
- Fiorica, C.; Palumbo, F.S.; Pitarresi, G.; Puleio, R.; Condorelli, L.; Collura, G.; Giammona, G. A hyaluronic acid/cyclodextrin based injectable hydrogel for local doxorubicin delivery to solid tumors. Int. J. Pharm. 2020, 589, 119879. [Google Scholar] [CrossRef]
- Gheysoori, P.; Paydayesh, A.; Jafari, M.; Peidayesh, H. Thermoresponsive nanocomposite hydrogels based on Gelatin/poly (N–isopropylacrylamide) (PNIPAM) for controlled drug delivery. Eur. Polym. J. 2023, 186, 111846. [Google Scholar] [CrossRef]
- Gallagher, S.; Florea, L.; Fraser, K.; Diamond, D. Swelling and Shrinking Properties of Thermo-Responsive Polymeric Ionic Liquid Hydrogels with Embedded Linear pNIPAAM. Int. J. Mol. Sci. 2014, 15, 5337–5349. [Google Scholar] [CrossRef]
- Wang, J.; Williamson, G.S.; Yang, H. Branched polyrotaxane hydrogels consisting of alpha-cyclodextrin and low-molecular-weight four-arm polyethylene glycol and the utility of their thixotropic property for controlled drug release. Colloids Surf. B Biointerfaces 2018, 165, 144–149. [Google Scholar] [CrossRef]
- Jiang, Y.; Meng, X.; Wu, Z.; Qi, X. Modified chitosan thermosensitive hydrogel enables sustained and efficient anti-tumor therapy via intratumoral injection. Carbohydr. Polym. 2016, 144, 245–253. [Google Scholar] [CrossRef]
- Yi, P.; Wang, Y.; He, P.; Zhan, Y.; Sun, Z.; Li, Y.; Zhang, Y. Study on β-cyclodextrin-complexed nanogels with improved thermal response for anticancer drug delivery. Mater. Sci. Eng. C 2017, 78, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Gami, P.; Kundu, D.; Seera, S.D.K.; Banerjee, T. Chemically crosslinked xylan–β-Cyclodextrin hydrogel for the in vitro delivery of curcumin and 5-Fluorouracil. Int. J. Biol. Macromol. 2020, 158, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Torchio, A.; Cassino, C.; Lavella, M.; Gallina, A.; Stefani, A.; Boffito, M.; Ciardelli, G. Injectable supramolecular hydrogels based on custom-made poly(ether urethane)s and α-cyclodextrins as efficient delivery vehicles of curcumin. Mater. Sci. Eng. C 2021, 127, 112194. [Google Scholar] [CrossRef] [PubMed]
- Almawash, S.; El Hamd, M.A.; Osman, S.K. Polymerized β-Cyclodextrin-Based Injectable Hydrogel for Sustained Release of 5-Fluorouracil/Methotrexate Mixture in Breast Cancer Management: In Vitro and In Vivo Analytical Validations. Pharmaceutics 2022, 14, 817. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Huang, Y.; Zong, S.; Jiang, J. Preparation and Drug Release Properties of a Thermo Sensitive GA Hydrogel. Polymers 2020, 13, 119. [Google Scholar] [CrossRef]
- Liu, L.; Li, L.; Qing, Y.; Yan, N.; Wu, Y.; Li, X.; Tian, C. Mechanically strong and thermosensitive hydrogels reinforced with cellulose nanofibrils. Polym. Chem. 2016, 7, 7142–7151. [Google Scholar] [CrossRef]
- Jung, Y.; Park, W.; Park, H.; Lee, D.-K.; Na, K. Thermo-sensitive injectable hydrogel based on the physical mixing of hyaluronic acid and Pluronic F-127 for sustained NSAID delivery. Carbohydr. Polym. 2017, 156, 403–408. [Google Scholar] [CrossRef]
- Wu, W.-X.; Huang, Y.-C.; Lee, W.-F. Effect of poly(ethylene glycol)-derived crosslinkers on the properties of thermosensitive hydrogels. Iran. Polym. J. 2020, 29, 679–691. [Google Scholar] [CrossRef]
- Thakur, S.; Singh, H.; Singh, A.; Kaur, S.; Sharma, A.; Singh, S.K.; Kaur, S.; Kaur, G.; Jain, S.K. Thermosensitive injectable hydrogel containing carboplatin loaded nanoparticles: A dual approach for sustained and localized delivery with improved safety and therapeutic efficacy. J. Drug Deliv. Sci. Technol. 2020, 58, 101817. [Google Scholar] [CrossRef]
- Seo, J.W.; Shin, S.R.; Lee, M.Y.; Cha, J.M.; Min, K.H.; Lee, S.C.; Shin, S.Y.; Bae, H. Injectable hydrogel derived from chitosan with tunable mechanical properties via hybrid-crosslinking system. Carbohydr. Polym. 2021, 251, 117036. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Luo, J.; Liu, Y.; Su, S.; Fu, S.; Yang, X.; Li, B. Intratumoral Administration of Thermosensitive Hydrogel Co-Loaded with Norcantharidin Nanoparticles and Doxorubicin for the Treatment of Hepatocellular Carcinoma. Int. J. Nanomed. 2021, 16, 4073–4085. [Google Scholar] [CrossRef]
- Zhao, B.; Zhou, B.; Shi, K.; Zhang, R.; Dong, C.; Xie, D.; Tang, L.; Tian, Y.; Qian, Z.; Yang, L. Sustained and targeted delivery of siRNA/DP7-C nanoparticles from injectable thermosensitive hydrogel for hepatocellular carcinoma therapy. Cancer Sci. 2021, 112, 2481–2492. [Google Scholar] [CrossRef] [PubMed]
- Dirauf, M.; Muljajew, I.; Weber, C.; Schubert, U.S. Recent advances in degradable synthetic polymers for biomedical applications—Beyond polyesters. Prog. Polym. Sci. 2022, 129, 101547. [Google Scholar] [CrossRef]
- Shen, W.; Chen, X.; Luan, J.; Wang, D.; Yu, L.; Ding, J. Sustained Codelivery of Cisplatin and Paclitaxel via an Injectable Prodrug Hydrogel for Ovarian Cancer Treatment. ACS Appl. Mater. Interfaces 2017, 9, 40031–40046. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, T.; Kageyama, T.; Ohta, S.; Fukuda, J.; Ito, T. Injectable Hydrogel with Slow Degradability Composed of Gelatin and Hyaluronic Acid Cross-Linked by Schiff’s Base Formation. Biomacromolecules 2018, 19, 288–297. [Google Scholar] [CrossRef]
- Wang, M.; Chen, M.; Niu, W.; Winston, D.D.; Cheng, W.; Lei, B. Injectable biodegradation-visual self-healing citrate hydrogel with high tissue penetration for microenvironment-responsive degradation and local tumor therapy. Biomaterials 2020, 261, 120301. [Google Scholar] [CrossRef]
- Ma, H.; He, C.; Cheng, Y.; Li, D.; Gong, Y.; Liu, J.; Tian, H.; Chen, X. PLK1shRNA and doxorubicin co-loaded thermosensitive PLGA-PEG-PLGA hydrogels for osteosarcoma treatment. Biomaterials 2014, 35, 8723–8734. [Google Scholar] [CrossRef]
- Cao, D.; Zhang, X.; Luo, Y.; Wu, H.; Ke, X.; Ci, T. Artificial Cells, Nanomedicine, and Biotechnology Liposomal doxorubicin loaded PLGA-PEG-PLGA based thermogel for sustained local drug delivery for the treatment of breast cancer Liposomal doxorubicin loaded PLGA-PEG-PLGA based thermogel for sustained local drug delivery for the treatment of breast cancer. Nanomed. Biotechnol. 2019, 47, 181–191. [Google Scholar] [CrossRef]
- Lv, Q.; He, C.; Quan, F.; Yu, S.; Chen, X. DOX/IL-2/IFN-γ co-loaded thermo-sensitive polypeptide hydrogel for efficient melanoma treatment. Bioact. Mater. 2018, 3, 118–128. [Google Scholar] [CrossRef]
- Cheng, Y.-E.; Wu, I.-E.; Chen, Y.-C.; Chu, I.-M. Thermo-Sensitive mPEG-PA-PLL Hydrogel for Drug Release of Calcitonin. Gels 2022, 8, 282. [Google Scholar] [CrossRef]
- Li, T.; Zhang, M.; Wang, J.; Wang, T.; Yao, Y.; Zhang, X.; Zhang, C.; Zhang, N. Thermosensitive Hydrogel Co-loaded with Gold Nanoparticles and Doxorubicin for Effective Chemoradiotherapy. AAPS J. 2016, 18, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Gao, W.; Hu, H.; Ma, K.; He, B.; Dai, W.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Novel thermo-sensitive hydrogel system with paclitaxel nanocrystals: High drug-loading, sustained drug release and extended local retention guaranteeing better efficacy and lower toxicity. J. Control. Release 2014, 174, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Turabee, M.H.; Jeong, T.H.; Ramalingam, P.; Kang, J.H.; Ko, Y.T. N,N,N-trimethyl chitosan embedded in situ Pluronic F127 hydrogel for the treatment of brain tumor. Carbohydr. Polym. 2019, 203, 302–309. [Google Scholar] [CrossRef]
- Ma, H.; He, C.; Cheng, Y.; Yang, Z.; Zang, J.; Liu, J.; Chen, X. Localized Co-delivery of Doxorubicin, Cisplatin, and Methotrexate by Thermosensitive Hydrogels for Enhanced Osteosarcoma Treatment. ACS Appl. Mater. Interfaces 2015, 7, 27040–27048. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Wang, Z.; Chen, B.; Dai, W.; Zhang, H.; He, B.; Wang, X.; Wang, Y.; Zhang, Q. Localized co-delivery of collagenase and trastuzumab by thermosensitive hydrogels for enhanced antitumor efficacy in human breast xenograft. Drug Deliv. 2018, 25, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Han, T.-S.; Hur, K.; Choi, B.; Lee, J.-Y.; Byeon, S.-J.; Min, J.; Yu, J.; Cho, J.-K.; Hong, J.; Lee, H.-J.; et al. Improvement of anti-cancer drug efficacy via thermosensitive hydrogel in peritoneal carcinomatosis in gastric cancer. Oncotarget 2017, 8, 108848–108858. [Google Scholar] [CrossRef]
- Watt, R.P.; Khatri, H.; Dibble, A.R.G. Injectability as a function of viscosity and dosing materials for subcutaneous administration. Int. J. Pharm. 2019, 554, 376–386. [Google Scholar] [CrossRef]
- Alonso, J.M.; Andrade del Olmo, J.; Perez Gonzalez, R.; Saez-Martinez, V. Injectable Hydrogels: From Laboratory to Industrialization. Polymers 2021, 13, 650. [Google Scholar] [CrossRef]
- Gong, J.; Wang, L.; Wu, J.; Yuan, Y.; Mu, R.J.; Du, Y.; Wu, C.; Pang, J. The rheological and physicochemical properties of a novel thermosensitive hydrogel based on konjac glucomannan/gum tragacanth. LWT 2019, 100, 271–277. [Google Scholar] [CrossRef]
- Xu, G.; Li, B.; Wang, T.; Wan, J.; Zhang, Y.; Huang, J.; Shen, Y. Enhancing the anti-ovarian cancer activity of quercetin using a self-assembling micelle and thermosensitive hydrogel drug delivery system. RSC Adv. 2018, 8, 21229–21242. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Yu, S.; Quan, F.; He, C.; Chen, X. Thermosensitive Polypeptide Hydrogels Co-Loaded with Two Anti-Tumor Agents to Reduce Multi-Drug Resistance and Enhance Local Tumor Treatment. Adv. Ther. 2020, 3, 1900165. [Google Scholar] [CrossRef]
- Gao, Y.; Ren, F.; Ding, B.; Sun, N.; Liu, X.; Ding, X.; Gao, S. A thermo-sensitive PLGA-PEG-PLGA hydrogel for sustained release of docetaxel. J. Drug Target. 2011, 19, 516–527. [Google Scholar] [CrossRef]
- Cho, H.; Gao, J.; Kwon, G.S. PEG-b-PLA micelles and PLGA-b-PEG-b-PLGA sol–gels for drug delivery. J. Control. Release 2016, 240, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Elstad, N.L.; Fowers, K.D. OncoGel (ReGel/paclitaxel)—Clinical applications for a novel paclitaxel delivery system. Adv. Drug Deliv. Rev. 2009, 61, 785–794. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Efficacy and Safety of OncoGelTM Added to Chemotherapy and Radiation before Surgery in Subjects with Esophageal Cancer. Available online: https://www.clinicaltrials.gov/ct2/show/NCT00573131#:~:text=OncoGel%20is%20a%20new%20experimental%20drug%20delivery%20system,to%206%20weeks%20as%20it%20releases%20the%20paclitaxel (accessed on 1 August 2022).
- DeWitt, J.M.; Murthy, S.K.; Ardhanari, R.; DuVall, G.A.; Wallner, G.; Litka, P.; Daugherty, C.; Fowers, K. EUS-guided paclitaxel injection as an adjunctive therapy to systemic chemotherapy and concurrent external beam radiation before surgery for localized or locoregional esophageal cancer: A multicenter prospective randomized trial. Gastrointest. Endosc. 2017, 86, 140–149. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Phase 1/2 Dose Escalation Study of Locally-Administered OncoGelTM in Subjects with Recurrent Glioma. Available online: https://clinicaltrials.gov/ct2/show/NCT00479765?term=oncogel&cond=Cancer&draw=2&rank=2 (accessed on 1 August 2022).
- ClinicalTrials.gov. The OLYMPUS Study—Optimized DeLivery of Mitomycin for Primary UTUC Study (Olympus). Available online: https://clinicaltrials.gov/ct2/show/NCT02793128?term=Mitomycin+gel&cond=upper+tract+urothelial+carcinoma&draw=2&rank=2 (accessed on 1 August 2022).
- Donin, N.M.; Duarte, S.; Lenis, A.T.; Caliliw, R.; Torres, C.; Smithson, A.; Strauss-Ayali, D.; Agmon-Gerstein, Y.; Malchi, N.; Said, J.; et al. Sustained-release Formulation of Mitomycin C to the Upper Urinary Tract Using a Thermosensitive Polymer: A Preclinical Study. Urology 2017, 99, 270–277. [Google Scholar] [CrossRef]
- Kleinmann, N.; Matin, S.F.; Pierorazio, P.M.; Gore, J.L.; Shabsigh, A.; Hu, B.; Chamie, K.; Godoy, G.; Hubosky, S.; Rivera, M.; et al. Primary chemoablation of low-grade upper tract urothelial carcinoma using UGN-101, a mitomycin-containing reverse thermal gel (OLYMPUS): An open-label, single-arm, phase 3 trial. Lancet Oncol. 2020, 21, 776–785. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Approves First Therapy for Treatment of Low-Grade Upper Tract Urothelial Cancer; US Food and Drug Administration: Silver Spring, MD, USA, 2020.
- ClinicalTrials.gov. Efficacy and Safety of UGN-101 in Recurrent Patients (Retreatment). Available online: https://clinicaltrials.gov/ct2/show/NCT04006691?term=Mitomycin+gel&cond=upper+tract+urothelial+carcinoma&draw=2&rank=1 (accessed on 1 August 2022).
- Chevli, K.K.; Shore, N.D.; Trainer, A.; Smith, A.B.; Saltzstein, D.; Ehrlich, Y.; Raman, J.D.; Friedman, B.; D’Anna, R.; Morris, D.; et al. Primary Chemoablation of Low-Grade Intermediate-Risk Nonmuscle-Invasive Bladder Cancer Using UGN-102, a Mitomycin-Containing Reverse Thermal Gel (Optima II): A Phase 2b, Open-Label, Single-Arm Trial. J. Urol. 2022, 207, 61–69. [Google Scholar] [CrossRef]
- Stover, A.M.; Basak, R.; Mueller, D.; Lipman, R.; Teal, R.; Hilton, A.; Giannone, K.; Waheed, M.; Smith, A.B. Minimal Patient-Reported Side Effects for a Chemoablative Gel (UGN-102) Used as Frontline Treatment in Adults with Nonmuscle-Invasive Bladder Cancer. J. Urol. 2022, 208, 580–588. [Google Scholar] [CrossRef]
- Luo, S.-D.; Wu, S.-C.; Chen, W.-C.; Wu, C.-N.; Chiu, T.-J.; Yang, Y.-H.; Li, S.-H.; Fang, F.-M.; Huang, T.-L.; Hsiao, C.-C.; et al. Low-dose aspirin confers a survival benefit in patients with pathological advanced-stage oral squamous cell carcinoma. Sci. Rep. 2021, 11, 17161. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.-P.; Huang, W.; Wang, X.; Xu, C.; Qin, W.-T.; Li, D.; Tian, G.; Li, Q.; Zhou, Y.; Chen, S.; et al. Terbinafine prevents colorectal cancer growth by inducing dNTP starvation and reducing immune suppression. Mol. Ther. 2022, 30, 3284–3299. [Google Scholar] [CrossRef] [PubMed]





| Polymer | Polymer Concentration in Aqueous Solution (% w/v) | LCST (°C) | Reference |
|---|---|---|---|
| Poly(N-isopropyl acrylamide), PNIPAM | ~2.5 | ~32 | [79] |
| Poly(vinyl methyl ether), PVME | ~5 | ~40 | [80] |
| PLGA-PEG-PLGA | ~25 | ~25 | [81] |
| Poly(N-vinylcaprolactam), PNVCL | ~0.5 | ~30 | [82] |
| Chitosan–glycerol phosphate | ~1 CH + ~10 GP | ~37 | [83] |
| Pluronic-F127, PF-127 | ~15 | ~25 | - |
| Hydroxypropyl methylcellulose, HPMC | ~1 | ~70 | [84] |
| Polyphosphazene derivatives | ~2 | 25–80 | [85] |
| Methoxy poly(ethylene glycol) (MPEG)–diblock copolymers) | ~1 | 32–42 | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanga, S.; Aucamp, M.; Ramburrun, P. Injectable Thermoresponsive Hydrogels for Cancer Therapy: Challenges and Prospects. Gels 2023, 9, 418. https://doi.org/10.3390/gels9050418
Tanga S, Aucamp M, Ramburrun P. Injectable Thermoresponsive Hydrogels for Cancer Therapy: Challenges and Prospects. Gels. 2023; 9(5):418. https://doi.org/10.3390/gels9050418
Chicago/Turabian StyleTanga, Sandrine, Marique Aucamp, and Poornima Ramburrun. 2023. "Injectable Thermoresponsive Hydrogels for Cancer Therapy: Challenges and Prospects" Gels 9, no. 5: 418. https://doi.org/10.3390/gels9050418