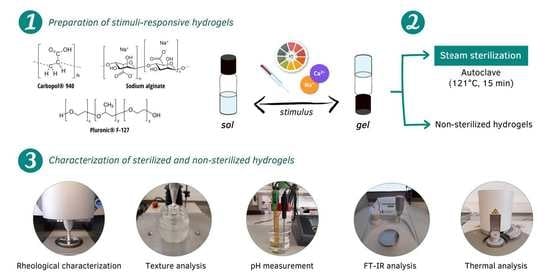
Effects of Steam Sterilization on the Properties of Stimuli-Responsive Polymer-Based Hydrogels
Abstract
:1. Introduction
1.1. Temperature-Responsive Polymers
1.2. pH-Responsive Polymers
1.3. Ionic Strength Responsive Polymers
2. Results and Discussion
2.1. Preparation of the Hydrogels
2.2. Characterization of the Hydrogels
2.2.1. Rheological Characterization
2.2.2. Texture Analysis
2.2.3. pH Measurement
2.2.4. Sol-Gel Transition
2.2.5. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.2.6. Differential Scanning Calorimetry (DSC)
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Hydrogels
4.3. Characterization of the Hydrogels
4.3.1. Rheological Characterization
4.3.2. Texture Analysis
4.3.3. pH Measurement
4.3.4. Sol-Gel Transition
4.3.5. Fourier-Transform Infrared (FT-IR) Spectroscopy
4.3.6. Differential Scanning Calorimetry (DSC)
4.3.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, X.; Hung, H.C.; Jain, P.; Sun, F.; Xu, X.; Yang, W.; Bai, T.; Jiang, S. Sterilization, hydration-dehydration and tube fabrication of zwitterionic hydrogels. Biointerphases 2017, 12, 02c411. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Khare, A.R. Preparation, structure and diffusional behavior of hydrogels in controlled release. Adv. Drug Deliv. Rev. 1993, 11, 1–35. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.; Kan, C.W.; Wang, W. Dual-responsive (pH/temperature) Pluronic F-127 hydrogel drug delivery system for textile-based transdermal therapy. Sci. Rep. 2019, 9, 11658. [Google Scholar] [CrossRef]
- Priya James, H.; John, R.; Alex, A.; Anoop, K.R. Smart polymers for the controlled delivery of drugs—A concise overview. Acta Pharm. Sin. B 2014, 4, 120–127. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chi-Leung Hui, P. Review of Stimuli-Responsive Polymers in Drug Delivery and Textile Application. Molecules 2019, 24, 2547. [Google Scholar] [CrossRef]
- Kwon, K.W.; Park, M.J.; Bae, Y.H.; Kim, H.D.; Char, K. Gelation behavior of PEO–PLGA–PEO triblock copolymers in water. Polymer 2002, 43, 3353–3358. [Google Scholar] [CrossRef]
- Escobar-Chávez, J.J.; López-Cervantes, M.; Naïk, A.; Kalia, Y.N.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J. Pharm. Pharm. Sci. 2006, 9, 339–358. [Google Scholar]
- Bazban-Shotorbani, S.; Hasani-Sadrabadi, M.M.; Karkhaneh, A.; Serpooshan, V.; Jacob, K.I.; Moshaverinia, A.; Mahmoudi, M. Revisiting structure-property relationship of pH-responsive polymers for drug delivery applications. J. Control. Release 2017, 253, 46–63. [Google Scholar] [CrossRef]
- Al-Kinani, A.A.; Zidan, G.; Elsaid, N.; Seyfoddin, A.; Alani, A.W.G.; Alany, R.G. Ophthalmic gels: Past, present and future. Adv. Drug Deliv. Rev. 2018, 126, 113–126. [Google Scholar] [CrossRef]
- Kushwaha, S.K.; Saxena, P.; Rai, A. Stimuli sensitive hydrogels for ophthalmic drug delivery: A review. Int. J. Pharm. Investig. 2012, 2, 54–60. [Google Scholar] [CrossRef]
- García, M.C. 14—Ionic-strength-responsive polymers for drug delivery applications. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Makhlouf, A.S.H., Abu-Thabit, N.Y., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 393–409. [Google Scholar]
- Galante, R.; Pinto, T.J.A.; Colaço, R.; Serro, A.P. Sterilization of hydrogels for biomedical applications: A review. J. Biomed. Mater. Res. B 2018, 106, 2472–2492. [Google Scholar] [CrossRef]
- Bento, C.S.A.; Gaspar, M.C.; Coimbra, P.; de Sousa, H.C.; Braga, M.E.M. A review of conventional and emerging technologies for hydrogels sterilization. Int. J. Pharm. 2023, 634, 122671. [Google Scholar] [CrossRef]
- Rizwan, M.; Chan, S.W.; Comeau, P.A.; Willett, T.L.; Yim, E.K.F. Effect of sterilization treatment on mechanical properties, biodegradation, bioactivity and printability of GelMA hydrogels. Biomed. Mater. 2020, 15, 065017. [Google Scholar] [CrossRef]
- Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 7, 2041731416648810. [Google Scholar] [CrossRef]
- Tao, M.; Ao, T.; Mao, X.; Yan, X.; Javed, R.; Hou, W.; Wang, Y.; Sun, C.; Lin, S.; Yu, T.; et al. Sterilization and disinfection methods for decellularized matrix materials: Review, consideration and proposal. Bioact. Mater. 2021, 6, 2927–2945. [Google Scholar] [CrossRef]
- Lohani, A.; Singh, G.; Bhattacharya, S.S.; Verma, A. Interpenetrating polymer networks as innovative drug delivery systems. J. Drug Deliv. 2014, 2014, 583612. [Google Scholar] [CrossRef]
- Dejeu, I.L.; Vicaș, L.G.; Vlaia, L.L.; Jurca, T.; Mureșan, M.E.; Pallag, A.; Coneac, G.H.; Olariu, I.V.; Muț, A.M.; Bodea, A.S.; et al. Study for Evaluation of Hydrogels after the Incorporation of Liposomes Embedded with Caffeic Acid. Pharmaceuticals 2022, 15, 175. [Google Scholar] [CrossRef]
- Amiji, M.M.; Lai, P.K.; Shenoy, D.B.; Rao, M. Intratumoral administration of paclitaxel in an in situ gelling poloxamer 407 formulation. Pharm. Dev. Technol. 2002, 7, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Gao, W.; Hu, H.; Ma, K.; He, B.; Dai, W.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Novel thermo-sensitive hydrogel system with paclitaxel nanocrystals: High drug-loading, sustained drug release and extended local retention guaranteeing better efficacy and lower toxicity. J. Control. Release 2014, 174, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.S.; Batista, J.V.C.; Melo, M.N.O.; Campos, V.E.B.; Toledo, A.; Oliveira, A.P.; Picciani, P.H.S.; Baumgartner, S.; Holandino, C. Pluronic(®) F127 Thermoresponsive Viscum album Hydrogel: Physicochemical Features and Cellular In Vitro Evaluation. Pharmaceutics 2022, 14, 2775. [Google Scholar] [CrossRef] [PubMed]
- Łabowska, M.B.; Skrodzka, M.; Sicińska, H.; Michalak, I.; Detyna, J. Influence of Cross-Linking Conditions on Drying Kinetics of Alginate Hydrogel. Gels 2023, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Moturi, V.; Lee, Y. Thixotropic property in pharmaceutical formulations. J. Control. Release 2009, 136, 88–98. [Google Scholar] [CrossRef]
- Sharpe, S.A.; Sandweiss, V.; Tuazon, J.; Giordano, M.; Witchey-Lakshmanan, L.; Hart, J.; Sequeira, J. Comparison of the flow properties of aqueous suspension corticosteroid nasal sprays under differing sampling conditions. Drug Dev. Ind. Pharm. 2003, 29, 1005–1012. [Google Scholar] [CrossRef]
- Silva, A.C.; Amaral, M.H.; González-Mira, E.; Santos, D.; Ferreira, D. Solid lipid nanoparticles (SLN)-based hydrogels as potential carriers for oral transmucosal delivery of risperidone: Preparation and characterization studies. Colloids Surf. B Biointerfaces 2012, 93, 241–248. [Google Scholar] [CrossRef]
- Beard, M.C.; Cobb, L.H.; Grant, C.S.; Varadarajan, A.; Henry, T.; Swanson, E.A.; Kundu, S.; Priddy, L.B. Autoclaving of Poloxamer 407 hydrogel and its use as a drug delivery vehicle. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 338–347. [Google Scholar] [CrossRef]
- Chansoria, P.; Narayanan, L.K.; Wood, M.; Alvarado, C.; Lin, A.; Shirwaiker, R.A. Effects of Autoclaving, EtOH, and UV Sterilization on the Chemical, Mechanical, Printability, and Biocompatibility Characteristics of Alginate. ACS Biomater. Sci. Eng. 2020, 6, 5191–5201. [Google Scholar] [CrossRef]
- Leo, W.J.; McLoughlin, A.J.; Malone, D.M. Effects of sterilization treatments on some properties of alginate solutions and gels. Biotechnol. Prog. 1990, 6, 51–53. [Google Scholar] [CrossRef]
- Wróblewska, M.; Szekalska, M.; Hafner, A.; Winnicka, K. Oleogels and bigels as topical drug carriers for ketoconazole ñ development and in vitro characterization. Acta Pol. Pharm. 2018, 75, 777–786. [Google Scholar]
- Almeida, H.; Lobão, P.; Frigerio, C.; Fonseca, J.; Silva, R.; Sousa Lobo, J.M.; Amaral, M.H. Preparation, characterization and biocompatibility studies of thermoresponsive eyedrops based on the combination of nanostructured lipid carriers (NLC) and the polymer Pluronic F-127 for controlled delivery of ibuprofen. Pharm. Dev. Technol. 2017, 22, 336–349. [Google Scholar] [CrossRef]
- England, R.J.; Homer, J.J.; Knight, L.C.; Ell, S.R. Nasal pH measurement: A reliable and repeatable parameter. Clin. Otolaryngol. Allied Sci. 1999, 24, 67–68. [Google Scholar] [CrossRef]
- Bermudez, J.M.; Grau, R. Thermosensitive poloxamer-based injectables as controlled drug release platforms for veterinary use: Development and in-vitro evaluation. Int. Res. J. Pharm. Pharmacol. 2011, 1, 109–118. [Google Scholar]
- Hyun, K.; Wilhelm, M.; Klein, C.O.; Cho, K.S.; Nam, J.G.; Ahn, K.H.; Lee, S.J.; Ewoldt, R.H.; McKinley, G.H. A review of nonlinear oscillatory shear tests: Analysis and application of large amplitude oscillatory shear (LAOS). Prog. Polym. Sci. 2011, 36, 1697–1753. [Google Scholar] [CrossRef]
- Zerbinati, N.; Capillo, M.C.; Sommatis, S.; Maccario, C.; Alonci, G.; Rauso, R.; Galadari, H.; Guida, S.; Mocchi, R. Rheological Investigation as Tool to Assess Physicochemical Stability of a Hyaluronic Acid Dermal Filler Cross-Linked with Polyethylene Glycol Diglycidyl Ether and Containing Calcium Hydroxyapatite, Glycine and L-Proline. Gels 2022, 8, 264. [Google Scholar] [CrossRef]
- Lippacher, A.; Müller, R.H.; Mäder, K. Liquid and semisolid SLN dispersions for topical application: Rheological characterization. Eur. J. Pharm. Biopharm. 2004, 58, 561–567. [Google Scholar] [CrossRef]
- NETZSCH White Paper—A Basic Introduction to Rheology. Available online: https://analyzing-testing.netzsch.com/en-US/products/rheometers/dynamic-shear-rheometer-dsr/kinexus-dsr (accessed on 15 March 2023).
- Sguizzato, M.; Valacchi, G.; Pecorelli, A.; Boldrini, P.; Simelière, F.; Huang, N.; Cortesi, R.; Esposito, E. Gallic acid loaded poloxamer gel as new adjuvant strategy for melanoma: A preliminary study. Colloids Surf. B Biointerfaces 2020, 185, 110613. [Google Scholar] [CrossRef]
- Chen, I.C.; Su, C.Y.; Chen, P.Y.; Hoang, T.C.; Tsou, Y.S.; Fang, H.W. Investigation and Characterization of Factors Affecting Rheological Properties of Poloxamer-Based Thermo-Sensitive Hydrogel. Polymers 2022, 14, 5353. [Google Scholar] [CrossRef]
- Kojarunchitt, T.; Hook, S.; Rizwan, S.; Rades, T.; Baldursdottir, S. Development and characterisation of modified poloxamer 407 thermoresponsive depot systems containing cubosomes. Int. J. Pharm. 2011, 408, 20–26. [Google Scholar] [CrossRef]
- Cho, S.H.; Lim, S.M.; Han, D.K.; Yuk, S.H.; Im, G.I.; Lee, J.H. Time-dependent alginate/polyvinyl alcohol hydrogels as injectable cell carriers. J. Biomater. Sci. Polym. Ed. 2009, 20, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Burak, J.; Grela, K.; Karolewicz, B.; Marciniak, D. Impact of sterilisation conditions on the rheological properties of thermoresponsive pluronic F-127-based gels for the ophthalmic use. Acta Pol. Pharm. 2018, 75, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Jagdale, S.; Pawar, S. Gellified Emulsion of Ofloxacin for Transdermal Drug Delivery System. Adv. Pharm. Bull. 2017, 7, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Karolewicz, B.; Gajda, M.; Górniak, A.; Owczarek, A.; Mucha, I. Pluronic F127 as a suitable carrier for preparing the imatinib base solid dispersions and its potential in development of a modified release dosage forms. J. Therm. Anal. Calorim. 2017, 130, 383–390. [Google Scholar] [CrossRef]
- Di Donato, C.; Iacovino, R.; Isernia, C.; Malgieri, G.; Varela-Garcia, A.; Concheiro, A.; Alvarez-Lorenzo, C. Polypseudorotaxanes of Pluronic® F127 with Combinations of α- and β-Cyclodextrins for Topical Formulation of Acyclovir. Nanomaterials 2020, 10, 613. [Google Scholar] [CrossRef]
- García-Couce, J.; Tomás, M.; Fuentes, G.; Que, I.; Almirall, A.; Cruz, L.J. Chitosan/Pluronic F127 Thermosensitive Hydrogel as an Injectable Dexamethasone Delivery Carrier. Gels 2022, 8, 44. [Google Scholar] [CrossRef]
- Flores-Hernández, C.G.; Cornejo-Villegas, M.L.A.; Moreno-Martell, A.; Del Real, A. Synthesis of a Biodegradable Polymer of Poly (Sodium Alginate/Ethyl Acrylate). Polymers 2021, 13, 504. [Google Scholar] [CrossRef]
- Abd-El Hafeez, S.I.; Eleraky, N.E.; Hafez, E.; Abouelmagd, S.A. Design and optimization of metformin hydrophobic ion pairs for efficient encapsulation in polymeric drug carriers. Sci. Rep. 2022, 12, 5737. [Google Scholar] [CrossRef]
- Kumar, P.; Mohan, C.; Kanamsrinivasan Uma Shankar, M.; Gulati, M. Physiochemical Characterization and Release Rate Studies of SolidDispersions of Ketoconazole with Pluronic F127 and PVP K-30. Iran. J. Pharm. Res. 2011, 10, 685–694. [Google Scholar]
- Aldawsari, M.F.; Ahmed, M.M.; Fatima, F.; Anwer, M.K.; Katakam, P.; Khan, A. Development and Characterization of Calcium-Alginate Beads of Apigenin: In Vitro Antitumor, Antibacterial, and Antioxidant Activities. Mar. Drugs 2021, 19, 467. [Google Scholar] [CrossRef]
- Almeida, H.; Lobão, P.; Frigerio, C.; Fonseca, J.; Silva, R.; Quaresma, P.; Sousa Lobo, J.M.; Amaral, M.H. Development of mucoadhesive and thermosensitive eyedrops to improve the ophthalmic bioavailability of ibuprofen. J. Drug Deliv. Sci. Technol. 2016, 35, 69–80. [Google Scholar] [CrossRef]
- Tuladhar, S.; Nelson, C.; Habib, A. Rheological Study of Highly Thixotropic Hydrogels for 3D BioPrinting Processes. In Proceedings of the 2021 IISE Annual Conference, Online, 20–25 May 2021. [Google Scholar]
- Marques, A.C.; Rocha, A.I.; Leal, P.; Estanqueiro, M.; Sousa Lobo, J.M. Development and characterization of mucoadhesive buccal gels containing lipid nanoparticles of ibuprofen. Int. J. Pharm. 2017, 533, 455–462. [Google Scholar] [CrossRef]
- Jones, D.S.; Lawlor, M.S.; Woolfson, A.D. Examination of the flow rheological and textural properties of polymer gels composed of poly(methylvinylether-co-maleic anhydride) and poly(vinylpyrrolidone): Rheological and mathematical interpretation of textural parameters. J. Pharm. Sci. 2002, 91, 2090–2101. [Google Scholar] [CrossRef]
- Panyamao, P.; Ruksiriwanich, W.; Sirisa-Ard, P.; Charumanee, S. Injectable Thermosensitive Chitosan/Pullulan-Based Hydrogels with Improved Mechanical Properties and Swelling Capacity. Polymers 2020, 12, 2514. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef]







| Composition | Hydrogels | ||
|---|---|---|---|
| C940 HG | PF127 HG | SA HG | |
| Carbopol® 940 | 0.5 | - | - |
| Pluronic® F-127 | - | 20.0 | - |
| Sodium alginate | - | - | 2.0 |
| Fenonip® | 0.1 | 0.1 | 0.1 |
| Triethanolamine | q.s. | - | - |
| Purified water | q.s. 100 | q.s. 100 | q.s. 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, I.; Marques, A.C.; Costa, P.C.; Amaral, M.H. Effects of Steam Sterilization on the Properties of Stimuli-Responsive Polymer-Based Hydrogels. Gels 2023, 9, 385. https://doi.org/10.3390/gels9050385
Ferreira I, Marques AC, Costa PC, Amaral MH. Effects of Steam Sterilization on the Properties of Stimuli-Responsive Polymer-Based Hydrogels. Gels. 2023; 9(5):385. https://doi.org/10.3390/gels9050385
Chicago/Turabian StyleFerreira, Inês, Ana Camila Marques, Paulo Cardoso Costa, and Maria Helena Amaral. 2023. "Effects of Steam Sterilization on the Properties of Stimuli-Responsive Polymer-Based Hydrogels" Gels 9, no. 5: 385. https://doi.org/10.3390/gels9050385