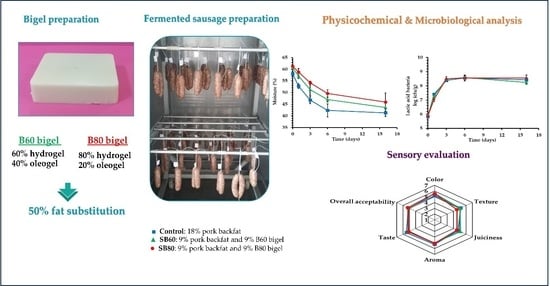
Bigels as Fat Replacers in Fermented Sausages: Physicochemical, Microbiological, Sensory, and Nutritional Characteristics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Analysis
2.1.1. Weight Loss
2.1.2. Moisture Content
2.1.3. Water Activity (αw)
2.1.4. pH
2.1.5. Lipid Oxidation
2.2. Microbiological Analysis
2.3. Sensory Evaluation
2.4. Nutritional Evaluation
3. Conclusions
4. Materials and Methods
4.1. Bigel Preparation
4.2. Sausage Preparation
4.3. Physicochemical Analysis
4.3.1. Weight Loss
4.3.2. Moisture
4.3.3. Water Activity (aw)
4.3.4. pH
4.3.5. Lipid Oxidation
4.4. Microbiological Analysis
4.5. Sensory Evaluation
4.6. Nutritional Profile Calculations
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holck, A.; Axelsson, L.; McLeod, A.; Rode, T.M.; Heir, E. Health and safety considerations of fermented sausages. J. Food Qual. 2017, 2017, 9753894. [Google Scholar] [CrossRef]
- Olivares, A.; Navarro, J.L.; Salvador, A.; Flores, M. Sensory acceptability of slow fermented sausages based on fat content and ripening time. Meat Sci. 2010, 86, 251–257. [Google Scholar] [CrossRef]
- Barbut, S. Reducing fats in processed meat products. In Processed Meats: Improving Safety, Nutrition and Quality; Kerry, J.P., Kerry, J.F., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2011; pp. 346–371. [Google Scholar] [CrossRef]
- Bolger, Z.; Brunton, N.P.; Lyng, J.G.; Monahan, F.J. Comminuted meat products—Consumption, composition, and approaches to healthier formulations. Food Rev. Int. 2017, 33, 143–166. [Google Scholar] [CrossRef]
- Ferro, A.C.; de Souza Paglarini, C.; Rodrigues Pollonio, M.A.; Lopes Cunha, R. Glyceryl monostearate-based oleogels as a new fat substitute in meat emulsion. Meat Sci. 2021, 174, 108424. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.J.; Vicente, A.A.; Cunha, R.L.; Cerqueira, M.A. Edible oleogels: An opportunity for fat replacement in foods. Food Funct. 2018, 9, 758–773. [Google Scholar] [CrossRef]
- Paglarini, C.D.S.; Vidal, V.A.S.; Ozaki, M.M.; Ribeiro, A.P.B.; Bernardinelli, O.D.; Camara, A.K.F.I.; Herrero, A.M.; Ruiz-Capillas, C.; Sabadini, E.; Pollonio, M.A.R. Inulin gelled emulsion as a fat replacer and fiber carrier in healthier Bologna sausage. Food Sci. Technol. Int. 2022, 28, 3–14. [Google Scholar] [CrossRef]
- Zampouni, K.; Soniadis, A.; Moschakis, T.; Biliaderis, C.G.; Lazaridou, A.; Katsanidis, E. Crystalline microstructure and physicochemical properties of olive oil oleogels formulated with monoglycerides and phytosterols. LWT-Food Sci. Technol. 2022, 154, 112815. [Google Scholar] [CrossRef]
- Kouzounis, D.; Lazaridou, A.; Katsanidis, E. Partial replacement of animal fat by oleogels structured with monoglycerides and phytosterols in frankfurter sausages. Meat Sci. 2017, 130, 38–46. [Google Scholar] [CrossRef]
- Panagiotopoulou, E.; Moschakis, T.; Katsanidis, E. Sunflower oil organogels and organogel-in-water emulsions (part II): Implementation in frankfurter sausages. LWT-Food Sci. Technol. 2016, 73, 351–356. [Google Scholar] [CrossRef]
- Zampouni, K.; Soniadis, A.; Dimakopoulou-Papazoglou, D.; Moschakis, T.; Biliaderis, C.G.; Katsanidis, E. Modified fermented sausages with olive oil oleogel and NaCl–KCl substitution for improved nutritional quality. LWT-Food Sci. Technol. 2022, 158, 113172. [Google Scholar] [CrossRef]
- Pintado, T.; Cofrades, S. Quality characteristics of healthy dry fermented sausages formulated with a mixture of olive and chia oil structured in oleogel or emulsion gel as animal fat replacer. Foods 2020, 9, 830. [Google Scholar] [CrossRef] [PubMed]
- Glisic, M.; Baltic, M.; Glisic, M.; Trbovic, D.; Jokanovic, M.; Parunovic, N.; Dimitrijevic, M.; Suvajdzic, B.; Boskovic, M.; Vasilev, D. Inulin-based emulsion-filled gel as a fat replacer in prebiotic- and PUFA-enriched dry fermented sausages. Int. J. Food Sci. 2019, 54, 787–797. [Google Scholar] [CrossRef]
- Martins, A.J.; Silva, P.; Maciel, F.; Pastrana, L.M.; Cunha, R.L.; Cerqueira, M.A.; Vicente, A.A. Hybrid gels: Influence of oleogel/hydrogel ratio on rheological and textural properties. Int. Food Res. J. 2019, 116, 1298–1305. [Google Scholar] [CrossRef]
- Shakeel, A.; Lupi, F.R.; Gabriele, D.; Baldino, N.; De Cindio, B. Bigels: A unique class of materials for drug delivery applications. Soft Mater. 2018, 16, 77–93. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Hydrogels as drug delivery systems; pros and cons. Trends Pharmacol. Sci. 2019, 5, 7–24. [Google Scholar] [CrossRef]
- Lv, X.; Huang, X.; Ma, B.; Chen, Y.; Batool, Z.; Fu, X.; Jin, Y. Modification methods and applications of egg protein gel properties: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2233–2252. [Google Scholar] [CrossRef]
- Singh, V.K.; Banerjee, I.; Agarwal, T.; Pramanik, K.; Bhattacharya, M.K.; Pal, K. Guar gum and sesame oil based novel bigels for controlled drug delivery. Colloids Surf. B 2014, 123, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.; Sagiri, S.S.; Pal, K.; Pramanik, K.; Rana, U.A.; Shakir, I.; Anis, A. Sunflower oil and protein-based novel bigels as matrices for drug delivery applications—Characterization and in vitro antimicrobial efficiency. Polym. Plast. Technol. Eng. 2015, 54, 837–850. [Google Scholar] [CrossRef]
- Paul, S.R.; Qureshi, D.; Yogalakshmi, Y.; Nayak, S.K.; Singh, V.K.; Syed, I.; Sarkar, P.; Pal, K. Development of bigels based on stearic acid–rice bran oil oleogels and tamarind gum hydrogels for controlled delivery applications. J. Surfactants Deterg. 2018, 21, 17–29. [Google Scholar] [CrossRef]
- Zampouni, K.; Mouzakitis, C.K.; Lazaridou, A.; Moschakis, T.; Katsanidis, E. Physicochemical properties and microstructure of bigels formed with gelatin and κ-carrageenan hydrogels and monoglycerides in olive oil oleogels. Food Hydrocoll. 2023, 140, 108636. [Google Scholar] [CrossRef]
- McClements, D.J. Food hydrocolloids: Application as functional ingredients to control lipid digestion and bioavailability. Food Hydrocoll. 2021, 111, 106404. [Google Scholar] [CrossRef]
- Zheng, H.; Mao, L.; Cui, M.; Liu, J.; Gao, Y. Development of food-grade bigels based on κ-carrageenan hydrogel and monoglyceride oleogels as carriers for β-carotene: Roles of oleogel fraction. Food Hydrocoll. 2020, 105, 105855. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, J.; Han, L.; Han, K.; Wei, W.; Wu, T.; Li, J.; Zhang, M. Development and characterization of novel bigels based on monoglyceride-beeswax oleogel and high acyl gellan gum hydrogel for lycopene delivery. Food Chem. 2021, 365, 130419. [Google Scholar] [CrossRef]
- Kanelaki, A.; Zampouni, K.; Mourtzinos, I.; Katsanidis, E. Hydrogels, oleogels and bigels as edible coatings of sardine fillets and delivery systems of rosemary extract. Gels 2022, 8, 660. [Google Scholar] [CrossRef] [PubMed]
- Bollom, M.A.; Clark, S.; Acevedo, N.C. Development and characterization of a novel soy lecithin-stearic acid and whey protein concentrate bigel system for potential edible applications. Food Hydrocoll. 2020, 101, 105570. [Google Scholar] [CrossRef]
- Fasolin, L.H.; Martins, A.J.; Cerqueira, M.A.; Vicente, A.A. Modulating process parameters to change physical properties of bigels for food applications. Food Struct. 2021, 28, 100173. [Google Scholar] [CrossRef]
- Quilaqueo, M.; Iturra, N.; Contardo, I.; Millao, S.; Morales, E.; Rubilar, M. Food-Grade Bigels with Potential to Replace Saturated and Trans Fats in Cookies. Gels 2022, 8, 445. [Google Scholar] [CrossRef]
- Álvarez, S.; Álvarez, C.; Hamill, R.; Mullen, A.M.; O’Neill, E. Drying dynamics of meat highlighting areas of relevance to dry-aging of beef. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5370–5392. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Triki, M.; Herrero, A.M.; Rodriguez-Salas, L.; Jiménez-Colmenero, F. Konjac gel as pork backfat replacer in dry fermented sausages: Processing and quality characteristics. Meat Sci. 2012, 92, 144–150. [Google Scholar] [CrossRef]
- Alejandre, M.; Poyato, C.; Ansorena, D.; Astiasarán, I. Linseed oil gelled emulsion: A successful fat replacer in dry fermented sausages. Meat Sci. 2016, 121, 107–113. [Google Scholar] [CrossRef]
- Bloukas, J.G.; Paneras, E.D.; Fournitzis, G.C. Effect of replacing pork backfat with olive oil on processing and quality characteristics of fermented sausages. Meat Sci. 1997, 45, 133–144. [Google Scholar] [CrossRef]
- Papadima, S.N.; Bloukas, J.G. Effect of fat level and storage conditions on quality characteristics of traditional Greek sausages. Meat Sci. 1999, 51, 103–113. [Google Scholar] [CrossRef]
- Feiner, G. (Ed.) Definitions of terms used in meat science and technology: Aw value (water activity). In Meat Products Handbook Practical Science and Technology; Woodhead Publishing Series in Food Science, Technology; Woodhead Publishing: Cambridge, UK, 2006; pp. 46–71. [Google Scholar]
- Chen, H.; Wu, D.; Ma, W.; Wu, C.; Tian, Y.; Wang, S.; Du, M. Strong fish gelatin hydrogels enhanced by carrageenan and potassium sulfate. Food Hydrocoll. 2021, 119, 106841. [Google Scholar] [CrossRef]
- Leistner, L.; Gorris, L.G. Food preservation by hurdle technology. Trends Food Sci. Technol. 1995, 6, 41–46. [Google Scholar] [CrossRef]
- Li, X.L.; Meng, R.; Xu, B.C.; Zhang, B.; Cui, B.; Wu, Z.Z. Function emulsion gels prepared with carrageenan and zein/carboxymethyl dextrin stabilized emulsion as a new fat replacer in sausages. Food Chem. 2022, 389, 133005. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Martins, A.J.; López-Pedrouso, M.; Cerqueira, M.A.; Purriños, L.; Pastrana, L.M.; Vicente, A.A.; Lorenzo, J.M. Evaluation of linseed oil oleogels to partially replace pork backfat in fermented sausages. J. Sci. Food Agric. 2020, 100, 218–224. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Y.; Li, X.; Liu, Y.; Yan, W. Effects of partial replacement of pork back fat by a camellia oil gel on certain quality characteristics of a cooked style Harbin sausage. Meat Sci. 2018, 146, 154–159. [Google Scholar] [CrossRef]
- Kanavouras, A.; Hernandez-Munoz, P.; Coutelieris, F.A. Packaging of olive oil: Quality issues and shelf-life predictions. Food Rev. Int. 2006, 22, 381–404. [Google Scholar] [CrossRef]
- Baka, A.M.; Papavergou, E.J.; Pragalaki, T.; Bloukas, J.G.; Kotzekidou, P. Effect of selected autochthonous starter cultures on processing and quality characteristics of Greek fermented sausages. LWT-Food Sci. Technol. 2011, 44, 54–61. [Google Scholar] [CrossRef]
- Drosinos, E.H.; Mataragas, M.; Xiraphi, N.; Moschonas, G.; Gaitis, F.; Metaxopoulos, J. Characterization of the microbial flora from a traditional Greek fermented sausage. Meat Sci. 2005, 69, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Mladenović, K.G.; Grujović, M.Ž.; Kiš, M.; Furmeg, S.; Tkalec, V.J.; Stefanović, O.D.; Kocić-Tanackov, S.D. Enterobacteriaceae in food safety with an emphasis on raw milk and meat. Appl. Microbiol. Biotechnol. 2021, 105, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- AOAC (Association of Official Analytical Chemists). AOAC: Official Methods of Analysis, 15th ed.; AOAC: Washington, DC, USA, 1990; Volume 1, pp. 69–90. [Google Scholar]
- Katsanidis, E.; Zampouni, K. Development of a novel steam distillation TBA test for the determination of lipid oxidation in meat products. Foods 2023, 12, 359. [Google Scholar] [CrossRef] [PubMed]
- United State Department of Agriculture. National Nutrient Database for Standard Reference Release. Available online: https://fdc.nal.usda.gov/ (accessed on 9 January 2023).




| Treatments | |||
|---|---|---|---|
| Nutrient | Control | SB60 | SB80 |
| Water (g) | 41.31 ± 1.40 a | 43.64 ± 2.21 a | 45.91 ± 3.10 a |
| Energy (kcal/kJ) | 376 ± 9 a/1578 ± 38 a | 342 ± 13 a/1438 ± 57 a | 320 ± 18 a/1342 ± 77 a |
| Total lipid (Fat) (g) | 30.75 ± 0.74 a | 26.12 ± 1.03 b | 23.42 ± 1.34 b |
| Fatty acids, total saturated (g) | 11.03 ± 0.26 a | 8.77 ± 0.34 b | 8.07 ± 0.46 b |
| Fatty acids, total monounsaturated (g) | 14.37 ± 0.34 a | 13.35 ± 0.52 ab | 11.49 ± 0.66 b |
| Fatty acids, total polyunsaturated (g) | 3.54 ± 0.08 a | 2.65 ± 0.10 b | 2.51 ± 0.14 b |
| Carbohydrate (g) | 1.70 ± 0.14 a | 1.95 ± 0.07 a | 1.99 ± 0.12 a |
| Fiber, total dietary (g) | 0.24 ± 0.00 a | 0.25 ± 0.01 a | 0.25 ± 0.01 a |
| Protein (g) | 21.45 ± 0.52 a | 23.29 ± 0.92 a | 23.80 ± 1.57 a |
| Sodium (mg) | 919 ± 22 a | 983 ± 39 a | 988 ± 57 a |
| Ash (g) | 1.39 ± 0.03 a | 1.41 ± 0.06 a | 1.42 ± 0.08 a |
| Cholesterol (mg) | 85.13 ± 2.04 a | 83.21 ± 3.27 a | 83.62 ± 4.80 a |
| Energy reduction (%) | 8.89 | 14.91 | |
| Total fat reduction (%) | 15.08 | 23.83 | |
| Saturated fatty acids reduction (%) | 20.53 | 26.82 | |
| Cholesterol reduction (%) | 2.25 | 1.77 | |
| Protein increase (%) | 8.57 | 11.00 | |
| Treatments | |||
|---|---|---|---|
| Ingredients | Control | SB60 | SB80 |
| Pork meat | 70.5 | 70.5 | 70.5 |
| Pork backfat | 18 | 9 | 9 |
| Bigel | 0 | 9 | 9 |
| Leek | 9 | 9 | 9 |
| NaCl | 1.5 | 1.5 | 1.5 |
| Spices | 0.99 | 0.99 | 0.99 |
| Starter culture | 0.01 | 0.01 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siachou, C.; Zampouni, K.; Katsanidis, E. Bigels as Fat Replacers in Fermented Sausages: Physicochemical, Microbiological, Sensory, and Nutritional Characteristics. Gels 2023, 9, 340. https://doi.org/10.3390/gels9040340
Siachou C, Zampouni K, Katsanidis E. Bigels as Fat Replacers in Fermented Sausages: Physicochemical, Microbiological, Sensory, and Nutritional Characteristics. Gels. 2023; 9(4):340. https://doi.org/10.3390/gels9040340
Chicago/Turabian StyleSiachou, Christina, Konstantina Zampouni, and Eugenios Katsanidis. 2023. "Bigels as Fat Replacers in Fermented Sausages: Physicochemical, Microbiological, Sensory, and Nutritional Characteristics" Gels 9, no. 4: 340. https://doi.org/10.3390/gels9040340