Characterization of a Bioink Combining Extracellular Matrix-like Hydrogel with Osteosarcoma Cells: Preliminary Results
Abstract
:1. Introduction
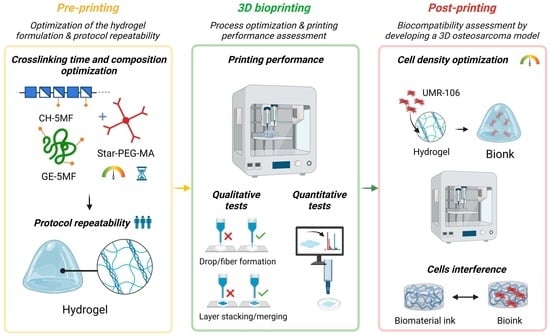
2. Results and Discussion
2.1. Layer Stacking or Merging: Temperature and Cross-Linking Time Effect
2.2. Drop or Fiber Formation: Cross-Linking Optimization
2.3. Quantitative Printing Performance: Operator and Protocol Repeatability
2.4. 3D Cultures
2.4.1. Microscopical Observation of Cell Growth in 3D Models
2.4.2. Cell Impact on Shape Fidelity and 3D Structures
2.5. Limitations
3. Conclusions
4. Materials and Methods
4.1. Hydrogel Formulation
4.2. Three-Dimensional Bioprinter
4.3. Bioprinter Set-Up
4.4. Scaffold Design and 3D Printing
4.5. Assessment of Printing Performance
4.5.1. Assessment of Printing Performance: Qualitative Protocol
Layer Stacking or Merging: Cross-Linking-Time and Printing-Temperature Effect
Drop or Fiber Formation: Cross-Linking Optimization
4.5.2. Assessment of Printing Performance: Quantitative Protocol
- Filament size: the homogeneity of the filaments was evaluated by measuring the filament diameter (, , , …, ; Figure 9bi,ii). Equal diameters denote a homogeneous filament. The filaments’ average size () was calculated as the ratio between the sum of the diameters taken from the image analysis in different positions of the construct () and the total number of measurements (
- Filament distance: the surface tension between the biomaterial and the printing support (in this case, the Petri dish), as well as between each layer of the material, can cause the merging of adjacent filaments (Figure 9bi,ii). Therefore, an important parameter to define the resolution of our material is the minimum distance that it is able to guarantee between the grid filaments. The mean distance ( between the filaments was calculated as the ratio between the sum of the distances taken from the analysis of images in different positions of the construct () and the total number of measurements (
- Pore geometry or printability index: the optimal shape of pore geometry is rectangular, which indicates the ideal filament (Figure 9bi,ii). This evaluation was performed using the printability index () [41], calculated as:where L and A are pore perimeter and area, respectively. The Pr index is higher than 1 for irregular structures, 1 in case of perfect square pores, and lower than 1 for circular pores.
Quantitative Printing Performance: Protocol and Operator Repeatability
4.6. Cell Culture
4.7. Three-Dimensional Bioprinting Procedure and Biocompatibility
4.8. Microscopy Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashammakhi, N.; Ahadian, S.; Xu, C.; Montazerian, H.; Ko, H.; Nasiri, R.; Barros, N.; Khademhosseini, A. Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs. Mater. Today Bio 2019, 1, 100008. [Google Scholar] [CrossRef]
- Bahcecioglu, G.; Basara, G.; Ellis, B.W.; Ren, X.; Zorlutuna, P. Breast cancer models: Engineering the tumor microenvironment. Acta Biomater. 2020, 106, 1–21. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.; Groll, J.; Hutmacher, D.W. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef]
- Neves, S.C.; Moroni, L.; Barrias, C.C.; Granja, P.L. Leveling up hydrogels: Hybrid systems in tissue engineering. Trends Biotechnol. 2020, 38, 292–315. [Google Scholar] [CrossRef]
- Ooi, H.W.; Hafeez, S.; Van Blitterswijk, C.A.; Moroni, L.; Baker, M.B. Hydrogels that listen to cells: A review of cell-responsive strategies in biomaterial design for tissue regeneration. Mater. Horiz. 2017, 4, 1020–1040. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Crook, J.M. 3D Bioprinting. In Methods in Molecular Biology; Humana: New York, NY, USA, 2020; Volume 2140. [Google Scholar]
- Park, J.; Lee, S.J.; Chung, S.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. Cell-laden 3D bioprinting hydrogel matrix depending on different compositions for soft tissue engineering: Characterization and evaluation. Mater. Sci. Eng. C 2017, 71, 678–684. [Google Scholar] [CrossRef]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef]
- Paxton, N.; Smolan, W.; Böck, T.; Melchels, F.; Groll, J.; Jungst, T. Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication 2017, 9, 044107. [Google Scholar] [CrossRef]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.; Hutmacher, D.W.; Melchels, F.P.; Klein, T.J.; Malda, J. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef]
- Gillispie, G.; Prim, P.; Copus, J.; Fisher, J.; Mikos, A.G.; Yoo, J.J.; Atala, A.; Lee, S.J. Assessment methodologies for extrusion-based bioink printability. Biofabrication 2020, 12, 022003. [Google Scholar] [CrossRef]
- Meng, Y.; Cao, J.; Chen, Y.; Yu, Y.; Ye, L. 3D printing of a poly (vinyl alcohol)-based nano-composite hydrogel as an artificial cartilage replacement and the improvement mechanism of printing accuracy. J. Mater. Chem. B 2020, 8, 677–690. [Google Scholar] [CrossRef]
- Gao, T.; Gillispie, G.J.; Copus, J.S.; Pr, A.K.; Seol, Y.J.; Atala, A.; Yoo, J.J.; Lee, S.J. Optimization of gelatin–alginate composite bioink printability using rheological parameters: A systematic approach. Biofabrication 2018, 10, 034106. [Google Scholar] [CrossRef]
- Soltan, N.; Ning, L.; Mohabatpour, F.; Papagerakis, P.; Chen, X. Printability and cell viability in bioprinting alginate dialdehyde-gelatin scaffolds. ACS Biomater. Sci. Eng. 2019, 5, 2976–2987. [Google Scholar] [CrossRef]
- Wang, L.; Xu, M.E.; Luo, L.; Zhou, Y.; Si, P. Iterative feedback bio-printing-derived cell-laden hydrogel scaffolds with optimal geometrical fidelity and cellular controllability. Sci. Rep. 2018, 8, 2802. [Google Scholar] [CrossRef]
- Ribeiro, A.; Blokzijl, M.M.; Levato, R.; Visser, C.W.; Castilho, M.; Hennink, W.E.; Vermonden, T.; Malda, J. Assessing bioink shape fidelity to aid material development in 3D bioprinting. Biofabrication 2017, 10, 014102. [Google Scholar] [CrossRef]
- Habib, A.; Sathish, V.; Mallik, S.; Khoda, B. 3D printability of alginate-carboxymethyl cellulose hydrogel. Materials 2018, 11, 454. [Google Scholar] [CrossRef]
- Freeman, F.E.; Kelly, D.J. Tuning alginate bioink stiffness and composition for controlled growth factor delivery and to spatially direct MSC fate within bioprinted tissues. Sci. Rep. 2017, 7, 17042. [Google Scholar] [CrossRef]
- Naghieh, S.; Karamooz-Ravari, M.R.; Sarker, M.D.; Karki, E.; Chen, X. Influence of crosslinking on the mechanical behavior of 3D printed alginate scaffolds: Experimental and numerical approaches. J. Mech. Behav. Biomed. Mater. 2018, 80, 111–118. [Google Scholar] [CrossRef]
- Shin, J.Y.; Yeo, Y.H.; Jeong, J.E.; Park, S.A.; Park, W.H. Dual-crosslinked methylcellulose hydrogels for 3D bioprinting applications. Carbohydr. Polym. 2020, 238, 116192. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, K.; Nie, J.; Sun, M.; Fu, J.; Wang, H.; He, Y. Modeling the printability of photocuring and strength adjustable hydrogel bioink during projection-based 3D bioprinting. Biofabrication 2021, 13, 035032. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Kim, T.G.; Kim, B.S.; Kim, S.W.; Kwon, S.M.; Cho, D.W. Tailoring mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced photo-crosslinking. Acta Biomater. 2016, 33, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Kajave, N.S.; Schmitt, T.; Nguyen, T.-U.; Kishore, V. Dual crosslinking strategy to generate mechanically viable cell-laden printable constructs using methacrylated collagen bioinks. Mater. Sci. Eng. C 2020, 107, 110290. [Google Scholar] [CrossRef]
- Sun, W.; Starly, B.; Daly, A.C.; Burdick, J.A.; Groll, J.; Skeldon, G.; Shu, W.; Sakai, Y.; Shinohara, M.; Nishikawa, M.; et al. The Bioprinting Roadmap. Biofabrication 2020, 12, 022002. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D.; Pashuck, E.T.; Stevens, M.M. Achieving controlled biomolecule–biomaterial conjugation. Chem. Rev. 2018, 118, 7702–7743. [Google Scholar] [CrossRef] [PubMed]
- West-Livingston, L.N.; Park, J.; Lee, S.J.; Atala, A.; Yoo, J.J. The role of the microenvironment in controlling the fate of bioprinted stem cells. Chem. Rev. 2020, 120, 11056–11092. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Ashammakhi, N.; Wu, X.Y.; Khademhosseini, A. Crosslinking strategies for 3D bioprinting of polymeric hydrogels. Small 2020, 16, e2002931. [Google Scholar] [CrossRef]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D extracellular matrix mimics: Fundamental concepts and role of materials chemistry to influence stem cell fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef]
- Cadamuro, F.; Russo, L.; Nicotra, F. Biomedical Hydrogels Fabricated Using Diels–Alder Crosslinking. EurJOC 2021, 2021, 374–382. [Google Scholar] [CrossRef]
- Magli, S.; Rossi, G.B.; Risi, G.; Bertini, S.; Cosentino, C.; Crippa, L.; Ballarini, E.; Cavaletti, G.; Piazza, L.; Masseroni, E.; et al. Design and Synthesis of Chitosan—Gelatin Hybrid Hydrogels for 3D Printable in vitro Models. Front. Chem. 2020, 8, 524. [Google Scholar] [CrossRef]
- Magli, S.; Rossi, L.; Consentino, C.; Bertini, S.; Nicotra, F.; Russo, L. Combined analytical approaches to standardize and characterize biomaterials formulations: Application to chitosan-gelatin cross-linked hydrogels. Biomolecules 2021, 11, 683. [Google Scholar] [CrossRef] [PubMed]
- Groll, J.; Burdick, J.A.; Cho, D.W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Juengst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A definition of bioinks and their distinction from biomaterial inks. Biofabrication 2018, 11, 013001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Oklu, R.; Dokmeci, M.R.; Khademhosseini, A. Three-dimensional bioprinting strategies for tissue engineering. Cold Spring Harb. Perspect. Med. 2018, 8, a025718. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of cell binding to collagen and gelatin: A study of the effect of 2D and 3D architecture and surface chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef]
- Liu, J.C.; Heilshorn, S.C.; Tirrell, D.A. Comparative cell response to artificial extracellular matrix proteins containing the RGD and CS5 cell-binding domains. Biomacromolecules 2004, 5, 497–504. [Google Scholar] [CrossRef]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics. Bioeng. Transl. Med. 2019, 4, 96–115. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Velasquillo-Martínez, C.; García-Carvajal, Z.Y. Composite hydrogels based on gelatin, chitosan and polyvinyl alcohol to biomedical applications: A review. Int. J. Polym. Mater. 2020, 69, 1–20. [Google Scholar] [CrossRef]
- Taiarol, L.; Bigogno, C.; Sesana, S.; Kravicz, M.; Viale, F.; Pozzi, E.; Monza, L.; Carozzi, V.A.; Meregalli, C.; Valtorta, S.; et al. Givinostat-Liposomes: Anti-Tumor Effect on 2D and 3D Glioblastoma Models and Pharmacokinetics. Cancers 2022, 14, 2978. [Google Scholar] [CrossRef]
- Usai, F.; Loi, G.; Scocozza, F.; Bellato, M.; Castagliuolo, I.; Conti, M.; Pasotti, L. Design and biofabrication of bacterial living materials with robust and multiplexed biosensing capabilities. Mater. Today Bio 2022, 18, 100526. [Google Scholar] [CrossRef]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and shape fidelity of bioinks in 3D bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Rutz, A.L.; Hyland, K.E.; Jakus, A.E.; Burghardt, W.R.; Shah, R.N. A multimaterial bioink method for 3D printing tunable, cell-compatible hydrogels. Adv. Mater. 2015, 27, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, A.M.; Synoradzki, K.; Firlej, W.; Bartnik, E.; Sobczuk, P.; Fiedorowicz, M.; Grieb, P.; Rutkowski, P. Molecular biology of osteosarcoma. Cancers 2020, 12, 2130. [Google Scholar] [CrossRef] [PubMed]











| Pressure (kPa) | Results | |
|---|---|---|
| Sample A | 30–55 | Hydrogel accumulation at the nozzle outlet |
| 55–80 | Irregular fiber formation | |
| 80–125 | Regular/smooth fiber formation | |
| Sample B | 30–55 | Hydrogel accumulation at the nozzle outlet |
| 55–80 | Irregular fiber formation | |
| 80–125 | Regular/smooth fiber formation | |
| Sample C | 30–90 | Hydrogel accumulation at the nozzle outlet |
| 90–105 | Irregular fiber formation and break | |
| 105–125 | Continuous irregular fiber formation |
| Three-Dimensional-Bioprinter Operational Variables | |
|---|---|
| Extrusion pressure (kPa) | 50–60 |
| Conical nozzle diameter (mm) | 0.41 |
| Printing speed (mm/min) | 450 |
| Printing temperature (°C) | RT—37 |
| Sample | GE-MF (mg) | CH-MF (mg) | Star-PEG-MA (mg) |
|---|---|---|---|
| A | 165 | 85 | 17.5 |
| B | 165 | 85 | 17.5 |
| C | 165 | 85 | 26.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loi, G.; Stucchi, G.; Scocozza, F.; Cansolino, L.; Cadamuro, F.; Delgrosso, E.; Riva, F.; Ferrari, C.; Russo, L.; Conti, M. Characterization of a Bioink Combining Extracellular Matrix-like Hydrogel with Osteosarcoma Cells: Preliminary Results. Gels 2023, 9, 129. https://doi.org/10.3390/gels9020129
Loi G, Stucchi G, Scocozza F, Cansolino L, Cadamuro F, Delgrosso E, Riva F, Ferrari C, Russo L, Conti M. Characterization of a Bioink Combining Extracellular Matrix-like Hydrogel with Osteosarcoma Cells: Preliminary Results. Gels. 2023; 9(2):129. https://doi.org/10.3390/gels9020129
Chicago/Turabian StyleLoi, Giada, Gaia Stucchi, Franca Scocozza, Laura Cansolino, Francesca Cadamuro, Elena Delgrosso, Federica Riva, Cinzia Ferrari, Laura Russo, and Michele Conti. 2023. "Characterization of a Bioink Combining Extracellular Matrix-like Hydrogel with Osteosarcoma Cells: Preliminary Results" Gels 9, no. 2: 129. https://doi.org/10.3390/gels9020129