Potential Application of Ovalbumin Gel Nanoparticles Loaded with Carvacrol in the Preservation of Fresh Pork
Abstract
:1. Introduction
2. Results and Discussion
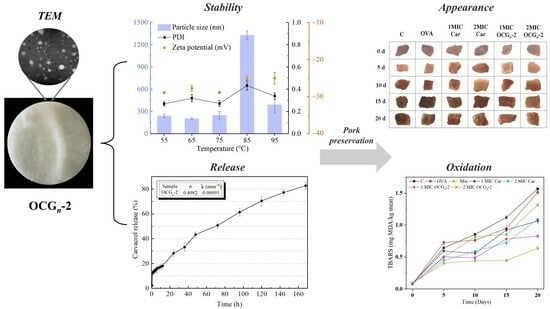
2.1. Form Analysis
2.2. Effects of Environmental Stresses on the Stability of OCGn-2
2.3. In Vitro Release Studies
2.4. Meat Storage Test
2.4.1. pH Test
2.4.2. Weight Loss Test
2.4.3. Color Measurement
2.4.4. Lipid Oxidation
2.4.5. Protein Oxidation
2.4.6. Microbiological Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Preparation of OCGn-2 Nanoparticles
4.3. Effect of Environmental Stress on the Stability of OCGn-2
4.3.1. Effect of Thermal Treatment
4.3.2. Effect of Ionic Strength
4.4. Transmission Electron Microscopy (TEM) Analysis
4.5. Particle Size and Polydispersity Index (PDI) Determination
4.6. In Vitro Release Studies
4.7. Application of Nanoparticles on Fresh Pork
4.7.1. Preparation of Fresh Pork Samples
4.7.2. pH Measurement
4.7.3. Weight Loss Test
4.7.4. Color Measurement
4.7.5. Lipid Oxidation
4.7.6. Protein Oxidation
4.7.7. Microbiological Analysis
4.7.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Yu, H.; Xie, Y.; Yang, F.; Yao, W. Screening of combination of essential oil components with synergistic inhibitory effect on quorum sensing with hexanal. J. Food Sci. Technol. 2022, 40, 72–81. [Google Scholar]
- Liu, J.; Fan, X.; Kong, B.; Wang, H. Effect of sodium alginate/crab shell powder bi-crosslinked water absorbent pad added with cinnamaldehyde on preservation of chilled meat. J. Food Sci. Technol. 2022, 40, 148–158. [Google Scholar]
- Imran, K.; Charles, N.T.; Deog-Hwan, O. Development and evaluation of chitosan and its derivative for the shelf life extension of beef meat under refrigeration storage. Int. J. Food Sci. Technol. 2017, 52, 1111–1121. [Google Scholar]
- Hussain, Z.; Li, X.; Zhang, D.; Hou, C.; Ijaz, M.; Bai, Y.; Xiao, X.; Zheng, X. Influence of adding cinnamon bark oil on meat quality of ground lamb during storage at 4 °C. Meat Sci. 2021, 171, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Eastwood, L.C.; Taylor, T.M.; Savell, J.W.; Gehring, K.B.; Arnold, A.N. Efficacy of antimicrobial interventions in reducing Salmonella enterica, Shiga toxin-producing Escherichia coli, Campylobacter, and Escherichia coli biotype I surrogates on non-chilled and chilled, skin-on and skinless pork. Meat Sci. 2020, 172, 108309. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.; Zhang, Y.; Mao, Y.; Yang, X.; Wang, X.; Luo, X.; Dong, P.; Zhu, L. Acid tolerance response of Salmonella during simulated chilled beef storage and its regulatory mechanism based on the PhoP/Q system. Food Microbiol. 2021, 95, 103716. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Jafari, S.M.; Aadil, R.M.; Assadpour, E.; Randhawa, M.A.; Mahmood, S. Development of active food packaging via incorporation of biopolymeric nanocarriers containing essential oils. Trends Food Sci. Technol. 2020, 101, 106–121. [Google Scholar] [CrossRef]
- Khaleque, M.A.; Keya, C.A.; Hasan, K.N.; Hoque, M.M.; Inatsu, Y.; Bari, M.L. Use of cloves and cinnamon essential oil to inactivate Listeria monocytogenes in ground beef at freezing and refrigeration temperatures. LWT-Food Sci. Technol. 2016, 74, 219–223. [Google Scholar] [CrossRef]
- Wang, L.; Liu, T.; Liu, L.; Liu, Y.; Wu, X. Impacts of chitosan nanoemulsions with thymol or thyme essential oil on volatile compounds and microbial diversity of refrigerated pork meat. Meat Sci. 2022, 185, 108706. [Google Scholar] [CrossRef]
- Tang, M.; Liu, F.; Wang, Q.; Wang, D.; Wang, D.; Zhu, Y.; Sun, Z.; Xu, W. Physicochemical characteristics of ginger essential oil nanoemulsion encapsulated by zein/NaCas and antimicrobial control on chilled chicken. Food Chem. 2022, 374, 131624. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.H.C. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des. 2008, 14, 3106–3199. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Ahmad, T.; Aadil, R.M.; Spotti, M.J.; Bakry, A.M.; Khan, I.M.; Zhao, L.; Riaz, T.; Tong, Q. Pectin polymers as wall materials for the nano-encapsulation of bioactive compounds. Trends Food Sci. Technol. 2019, 90, 35–46. [Google Scholar] [CrossRef]
- Abaee, A.; Mohammadian, M.; Jafari, S.M. Whey and soy protein-based hydrogels and nano-hydrogels as bioactive delivery systems. Trends Food Sci. Technol. 2017, 70, 69–81. [Google Scholar] [CrossRef]
- Hajebi, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Roghani-Mamaqani, H.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019, 92, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, J.; Yan, J.; Zhang, S.; Shi, C.; McClements, D.J.; Liu, X.; Liu, F. Development of antibacterial nanoemulsions incorporating thyme oil: Layer-by-layer self-assembly of whey protein isolate and chitosan hydrochloride. Food Chem. 2020, 339, 128016. [Google Scholar] [CrossRef] [PubMed]
- Joye, I.J.; Nelis, V.A.; McClements, D.J. Gliadin-based nanoparticles: Stabilization by post-production polysaccharide coating. Food Hydrocoll. 2015, 43, 236–242. [Google Scholar] [CrossRef]
- Veneranda, M.; Hu, Q.; Wang, T.; Luo, Y.; Castro, K.; Madariaga, J.M. Formation and characterization of zein-caseinate-pectin complex nanoparticles for encapsulation of eugenol. LWT-Food Sci. Technol. 2018, 89, 596–603. [Google Scholar] [CrossRef]
- Niu, F.G.; Pan, W.C.; Su, Y.J.; Yang, Y.J. Physical and antimicrobial properties of thyme oil emulsions stabilized by ovalbumin and gum arabic. Food Chem. 2016, 212, 138–145. [Google Scholar] [CrossRef]
- Rao, S.Q.; Xu, G.W.; Lu, X.N.; Zhang, R.; Gao, L.; Wang, Q.; Yang, Z.; Jiao, X. Characterization of ovalbumin-carvacrol inclusion complexes as delivery systems with antibacterial application. Food Hydrocoll. 2020, 105, 105753. [Google Scholar] [CrossRef]
- Rao, S.Q.; Xu, G.W.; Zeng, H.W.; Zheng, X.F.; Hu, Q.; Wang, Q.Y.; Yang, Z.Q.; Jiao, X.A. Physicochemical and antibacterial properties of fabricated ovalbumin-carvacrol gel nanoparticles. Food Funct. 2020, 11, 5133–5141. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.S.; Cao, D.D.; Ji, S.P.; Zhang, X.M.; Muhoza, B. Tannic acid-assisted cross-linked nanoparticles as a delivery system of eugenol: The characterization, thermal degradation and antioxidant properties. Food Hydrocoll. 2020, 104, 105717. [Google Scholar] [CrossRef]
- Wu, W.; Kong, X.; Zhang, C.; Hua, Y.; Chen, Y. Improving the stability of wheat gliadin nanoparticles—Effect of gum arabic addition. Food Hydrocoll. 2018, 80, 78–87. [Google Scholar] [CrossRef]
- Yu, N.; Shao, S.; Huan, W.; Ye, Q.; Nie, X.; Lu, Y.; Meng, X. Preparation of novel self-assembled albumin nanoparticles from Camellia seed cake waste for lutein delivery. Food Chem. 2022, 389, 133032. [Google Scholar] [CrossRef] [PubMed]
- Woranuch, S.; Yoksan, R. Eugenol-loaded chitosan nanoparticles: I. Thermal stability improvement of eugenol through encapsulation. Carbohydr. Polym. 2013, 96, 578–585. [Google Scholar] [CrossRef]
- Keawchaoon, L.; Yoksan, R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf. B Biointerfaces 2011, 84, 163–171. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent pH indicator film composed of agar/potato starch and anthocyanin extracts from purple sweet potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef]
- Pearce, K.L.; Rosenvold, K.; Andersen, H.J.; Hopkins, D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes—A review. Meat Sci. 2011, 89, 111–124. [Google Scholar] [CrossRef]
- Krishnan, K.R.; Babuskin, S.; Babu, P.A.S.; Sasikala, M.; Sabina, K.; Archana, G.; Sivarajan, M.; Sukumar, M. Antimicrobial and antioxidant effects of spice extracts on the shelf life extension of raw chicken meat. Int. J. Food Microbiol. 2014, 171, 32–40. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Fang, Z.; Lin, D.; Warner, R.D.; Ha, M. Effect of gallic acid/chitosan coating on fresh pork quality in modified atmosphere packaging. Food Chem. 2018, 260, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.D.P.P.; Freire, M.T.D.A.; Paula, E.S.M.D.; Kanashiro, A.L.S.; Catunda, F.A.P.; Rosa, A.F.; Balieiro, J.C.D.C.; Trindade, M.A. Stability of lamb loin stored under refrigeration and packed in different modified atmosphere packaging systems. Meat Sci. 2014, 96, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.-C.; Lee, S.-Y.; Yoon, J.-H.; Hong, K.-P.; Kang, Y.-S.; Park, S.-R.; Park, S.K.; Ha, S.-D.; Kim, G.-H.; Bae, D.-H. Inhibition of pork and fish oxidation by a novel plastic film coated with horseradish extract. LWT-Food Sci. Technol. 2008, 42, 856–861. [Google Scholar] [CrossRef]
- Huang, L.; Kong, B.H.; Zhao, J.Y.; Liu, Q.; Diao, X.P. Contributions of fat content and oxidation to the changes in physicochemical and sensory attributes of pork dumpling filler during frozen storage. J. Agric. Food Chem. 2014, 62, 6390–6399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liang, Y.; Li, X.; Kang, H. Effect of chitosan-gelatin coating containing nano-encapsulated tarragon essential oil on the preservation of pork slices. Meat Sci. 2020, 166, 108137. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-K.; Choi, J.S.; Yang, H.-S.; Park, T.-S.; Yim, D.-G. Natural curing agents as nitrite alternatives and their effects on the physicochemical, microbiological properties and sensory evaluation of sausages during storage. Meat Sci. 2018, 146, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Li, S.; Warner, R.D.; Fang, Z. Effect of oregano essential oil and resveratrol nanoemulsion loaded pectin edible coating on the preservation of pork loin in modified atmosphere packaging. Food Control 2020, 114, 107226. [Google Scholar] [CrossRef]
- Pateiro, M.; Vargas, F.C.; Chincha, A.A.I.A.; Sant, A.S.; Strozzi, I.; Rocchetti, G.; Barba, F.J.; Domínguez, R.; Lucini, L.; Sobral, P.J.D.A.; et al. Guarana seed extracts as a useful strategy to extend the shelf life of pork patties: UHPLC-ESI/QTOF phenolic profile and impact on microbial inactivation, lipid and protein oxidation and antioxidant capacity. Food Res. Int. 2018, 114, 55–63. [Google Scholar] [CrossRef]
- Huang, X.W.; Zou, X.B.; Shi, J.Y.; Guo, Y.N.; Zhao, J.W.; Zhang, J.C.; Hao, L.M. Determination of pork spoilage by colorimetric gas sensor array based on natural pigments. Food Chem. 2014, 145, 549–554. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, X.; Wang, L.; Wu, S.; Li, D.; Liu, C.; Feng, Z. Fabrication and characterization of a microemulsion stabilized by integrated phosvitin and gallic acid. J. Agric. Food Chem. 2020, 68, 5437–5447. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in vitro release study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Warner, R.D.; Fang, Z. Effect of chitosan/nisin/gallic acid coating on preservation of pork loin in high oxygen modified atmosphere packaging. Food Control 2019, 101, 9–16. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Collins, D.; Kilgannon, A.K.; Hopkins, D.L. The effect of technical replicate (repeats) on Nix Pro Color Sensor™ measurement precision for meat: A case-study on aged beef colour stability. Meat Sci. 2018, 135, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, G.; Jorgensen, S.S. A critical examination of some experimental variables in the 2-thiobarbituric acid (TBA) test for lipid oxidation in meat products. Z. Für Lebensm. Unters. Und-Forsch. 1996, 202, 205–210. [Google Scholar] [CrossRef]
- Bao, Y.; Ertbjerg, P. Relationship between oxygen concentration, shear force and protein oxidation in modified atmosphere packaged pork. Meat Sci. 2015, 110, 174–179. [Google Scholar] [CrossRef]









| Attributes | Treatment Groups | Storage Days | ||||
|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | ||
| L* | C | 36.38 ± 1.31 b | 35.29 ± 1.87 b | 36.74 ± 0.94 b | 39.44 ± 1.61 b | 46.53 ± 0.92 a |
| OVA | 40.00 ± 1.47 b | 40.43 ± 1.33 b | 42.45 ± 0.73 b | 42.98 ± 0.62 b | 45.44 ± 0.81 a | |
| Mix | 37.23 ± 2.12 b | 39.11 ± 1.03 b | 39.98 ± 0.75 b | 45.57 ± 0.78 a | 44.12 ± 1.51 a | |
| 1MIC Car | 33.73 ± 1.12 b | 36.86 ± 1.69 ab | 40.41 ± 1.38 a | 40.87 ± 0.82 a | 42.46 ± 1.15 a | |
| 2MIC Car | 40.66 ± 1.17 b | 41.96 ± 0.95 b | 40.31 ± 2.12 b | 49.07 ± 0.76 a | 48.50 ± 1.73 a | |
| 1MIC OCGn-2 | 45.09 ± 0.83 b | 43.21 ± 0.98 b | 44.16 ± 2.31 b | 37.08 ± 2.27 c | 50.23 ± 1.93 a | |
| 2MIC OCGn-2 | 51.20 ± 1.12 a | 44.89 ± 1.83 b | 48.97 ± 0.95 a | 49.05 ± 1.83 a | 48.45 ± 0.77 a | |
| a* | C | 11.90 ± 0.61 a | 10.47 ± 0.77 b | 9.01 ± 0.22 c | 4.25 ± 0.34 d | 3.21 ± 0.37 e |
| OVA | 10.81 ± 0.38 a | 7.26 ± 0.37 c | 9.14 ± 0.16 b | 6.04 ± 0.33 d | 4.15 ± 0.12 e | |
| Mix | 11.24 ± 0.38 a | 9.73 ± 0.22 b | 7.35 ± 0.28 c | 5.85 ± 0.87 d | 3.29 ± 0.11 e | |
| 1MIC Car | 11.75 ± 0.46 a | 10.64 ± 0.25 b | 7.23 ± 0.60 c | 5.73 ± 0.38 d | 3.77 ± 0.16 e | |
| 2MIC Car | 10.83 ± 0.13 a | 9.46 ± 0.76 b | 8.96 ± 0.39 b | 7.18 ± 0.19 c | 4.06 ± 0.15 d | |
| 1MIC OCGn-2 | 14.23 ± 0.33 a | 13.44 ± 0.44 a | 9.66 ± 0.17 b | 5.84 ± 0.17 c | 3.24 ± 0.11 d | |
| 2MIC OCGn-2 | 15.23 ± 0.41 a | 14.89 ± 0.61 a | 14.05 ± 0.44 a | 9.38 ± 0.28 b | 8.91 ± 0.12 b | |
| b* | C | 7.67 ± 0.46 c | 7.24 ± 0.51 c | 7.79 ± 0.11 c | 9.12 ± 0.49 b | 11.72 ± 0.72 a |
| OVA | 8.88 ± 0.52 b | 8.83 ± 0.32 b | 9.60 ± 0.37 b | 10.70 ± 0.74 ab | 11.80 ± 0.49 a | |
| Mix | 9.12 ± 0.42 b | 9.89 ± 0.42 b | 10.27 ± 0.49 b | 11.29 ± 0.53 ab | 12.10 ± 0.22 a | |
| 1MIC Car | 9.46 ± 0.67 b | 9.72 ± 0.14 b | 10.02 ± 0.23 b | 11.70 ± 0.75 a | 12.60 ± 0.77 a | |
| 2MIC Car | 8.23 ± 0.45 b | 8.41 ± 0.28 b | 8.39 ± 0.18 b | 9.84 ± 0.36 a | 10.43 ± 0.41 a | |
| 1MIC OCGn-2 | 12.38 ± 0.49 b | 13.30 ± 0.57 b | 13.53 ± 0.63 b | 14.2 ± 0.82 ab | 15.44 ± 0.43 a | |
| 2MIC OCGn-2 | 8.02 ± 0.13 c | 8.93 ± 0.31 b | 8.84 ± 0.18 b | 9.07 ± 0.31 b | 10.22 ± 0.27 a | |
| Treatment | Abbreviation | Nanoparticle (mg/mL) | OVA (mg/mL) | Car (mg/mL) |
|---|---|---|---|---|
| untreated samples | C | 0 | 0 | 0 |
| samples coated with OVA | OVA | 0 | 0.24 | 0 |
| samples coated with a physical mixture of OVA and Car | Mix | 0 | 0.24 | 0.16 |
| samples coated with 1 MIC free Car solution | 1MIC Car | 0 | 0 | 0.39 |
| samples coated with 2 MIC free Car solution | 2MIC Car | 0 | 0 | 0.78 |
| samples coated with 1 MIC OCGn-2 solution | 1MIC OCGn-2 | 0.40 | 0.24 | 0.16 |
| samples coated with 2 MIC OCGn-2 solution | 2MIC OCGn-2 | 0.80 | 0.48 | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Xu, G.; Su, Y.; Rao, S. Potential Application of Ovalbumin Gel Nanoparticles Loaded with Carvacrol in the Preservation of Fresh Pork. Gels 2023, 9, 941. https://doi.org/10.3390/gels9120941
Zhang R, Xu G, Su Y, Rao S. Potential Application of Ovalbumin Gel Nanoparticles Loaded with Carvacrol in the Preservation of Fresh Pork. Gels. 2023; 9(12):941. https://doi.org/10.3390/gels9120941
Chicago/Turabian StyleZhang, Ruyi, Guangwei Xu, Yujie Su, and Shengqi Rao. 2023. "Potential Application of Ovalbumin Gel Nanoparticles Loaded with Carvacrol in the Preservation of Fresh Pork" Gels 9, no. 12: 941. https://doi.org/10.3390/gels9120941