Biosynthesis of Peptide Hydrogel–Titania Nanoparticle Composites with Antibacterial Properties
Abstract
:1. Introduction
2. Results and Discussion
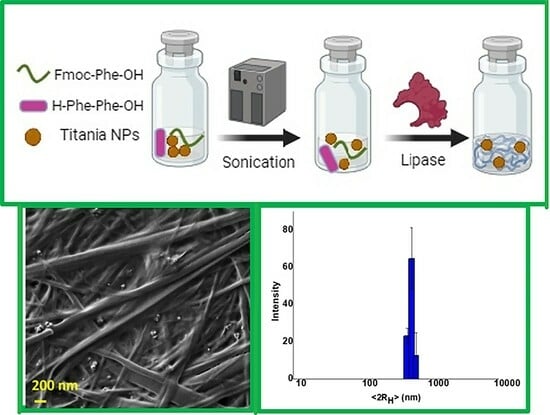
2.1. Preparation of Hgel-TiO2 Composites
2.2. Dynamic Light Scattering (DLS)
2.3. X-ray Small-Angle Scattering (SAXS)
2.4. Rheological Studies
2.5. Swelling Ability
2.6. Antibacterial Studies
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Hydrogel Composites
4.3. Field Emission Scanning Electron Microscopy (FESEM) Measurements
4.4. Dynamic Light Scattering (DLS)
4.5. Small-Angle X-ray Scattering (SAXS)
4.6. Rheological Measurements
4.7. Swelling Ability
4.8. Tested Microorganisms
4.9. Determination of Minimal Inhibitory Concentration (MIC)
4.10. Determination of Metabolic Activity
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology 2005, 16, 2346. [Google Scholar] [CrossRef]
- Jackson, N.; Hill, I.; Alhussan, A.; Bromma, K.; Morgan, J.; Abousaida, B.; Zahra, Y.; Mackeyev, Y.; Beckham, W.; Herchko, S.; et al. Dual Enhancement in the Radiosensitivity of Prostate Cancer through Nanoparticles and Chemotherapeutics. Cancer Nanotechnol. 2023, 14, 75. [Google Scholar] [CrossRef]
- Goel, M.; Mackeyev, Y.; Krishnan, S. Radiolabeled Nanomaterial for Cancer Diagnostics and Therapeutics: Principles and Concepts. Cancer Nanotechnol. 2023, 14, 15. [Google Scholar] [CrossRef]
- Suri, S.S.; Fenniri, H.; Singh, B. Nanotechnology-Based Drug Delivery Systems. J. Occup. Med. Toxicol. 2007, 2, 16. [Google Scholar] [CrossRef]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal Oxide Nanoparticles as Bactericidal Agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Sundaram, R.; Gokulakrishnan, R.; Selvanathan, K.; Selvam, D.S. Antibacterial Activity of Metal Oxide Nanoparticles against Opthalmic Pathogens. Int. J. Pharm. Res. Dev. 2011, 3, 122–127. [Google Scholar]
- Rajendra, R.; Balakumar, C.; Ahammed, H.; Jayakumar, S.; Vaideki, K.; Rajesh, E. Use of Zinc Oxide Nano Particles for Production of Antimicrobial Textiles. Int. J. Eng. Sci. Technol. 2010, 2, 202–208. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced Bioactivity of ZnO Nanoparticles-an Antimicrobial Study. Sci. Technol. Adv. Mater. 2008, 9, 35004. [Google Scholar] [CrossRef]
- Hosseinkhani, P.; Zand, A.; Imani, S.; Rezayi, M.; Rezaei-Zarchi, S. Determining the Antibacterial Effect of ZnO Nanoparticle against the Pathogenic Bacterium, Shigella Dysenteriae (Type 1). Int. J. Nano Dimens. 2011, 1, 279–285. [Google Scholar]
- Jadhav, S.; Gaikwad, S.; Nimse, M.; Rajbhoj, A. Copper Oxide Nanoparticles: Synthesis, Characterization and Their Antibacterial Activity. J. Clust. Sci. 2011, 22, 121–129. [Google Scholar] [CrossRef]
- Hajareh Haghighi, F.; Mercurio, M.; Cerra, S.; Salamone, T.A.; Binaymotlagh, R.; Palocci, C.; Romano Spica, V.; Fratoddi, I. Surface Modification of TiO2 Nanoparticles with Organic Molecules and Their Biological Applications. J. Mater. Chem. B 2023, 11, 2334–2366. [Google Scholar] [CrossRef]
- Garcia-Contreras, R.; Scougall-Vilchis, R.J.; Contreras-Bulnes, R.; Sakagami, H.; Morales-Luckie, R.A.; Nakajima, H. Mechanical, Antibacterial and Bond Strength Properties of Nano-Titanium-Enriched Glass Ionomer Cement. J. Appl. Oral Sci. 2015, 23, 321–328. [Google Scholar] [CrossRef]
- Soo, J.Z.; Chai, L.C.; Ang, B.C.; Ong, B.H. Enhancing the Antibacterial Performance of Titanium Dioxide Nanofibers by Coating with Silver Nanoparticles. ACS Appl. Nano Mater. 2020, 3, 5743–5751. [Google Scholar] [CrossRef]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef]
- Prokopyuk, V.; Yefimova, S.; Onishchenko, A.; Kapustnik, V.; Myasoedov, V.; Maksimchuk, P.; Butov, D.; Bespalova, I.; Tkachenko, A. Assessing the Cytotoxicity of TiO2−x Nanoparticles with a Different Ti3+(Ti2+)/Ti4+ Ratio. Biol. Trace Elem. Res. 2022, 201, 3117–3130. [Google Scholar] [CrossRef]
- Mungkalasiri, J.; Bedel, L.; Emieux, F.; Dore, J.; Renaud, F.N.R.; Sarantopoulos, C.; Maury, F. CVD Elaboration of Nanostructured TiO2-Ag Thin Films with Efficient Antibacterial Properties. Chem. Vap. Depos. 2010, 16, 35–41. [Google Scholar] [CrossRef]
- Lydakis-Simantiris, N.; Riga, D.; Katsivela, E.; Mantzavinos, D.; Xekoukoulotakis, N.P. Disinfection of Spring Water and Secondary Treated Municipal Wastewater by TiO2 Photocatalysis. Desalination 2010, 250, 351–355. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Sun, S.; Zhang, A.; Liu, Y. Binding Investigation on the Interaction between Methylene Blue (MB)/TiO2 Nanocomposites and Bovine Serum Albumin by Resonance Light-Scattering (RLS) Technique and Fluorescence Spectroscopy. J. Photochem. Photobiol. B Biol. 2013, 128, 12–19. [Google Scholar] [CrossRef]
- Gaëlle, C.; Erwann, H.; Saïd, E.; Maxime, E.; Marie-Claire, L.; Peter, H.; Jean-Pierre, G.; Valérie, K.; Nicolas, K.; Philippe, A. TiO2 Photocatalysis Damages Lipids and Proteins in Escherichia coli. Appl. Environ. Microbiol. 2014, 80, 2573–2581. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Hu, Y.; Chen, A.; Zhou, L.; Gao, H.; Liu, Y.; Liu, S. Study on Photocatalytic Antibacterial and Sustained-Release Properties of Cellulose/TiO2/β-CD Composite Hydrogel. J. Nanomater. 2019, 2019, 2326042. [Google Scholar] [CrossRef]
- Gabrielyan, L.; Hovhannisyan, A.; Gevorgyan, V.; Ananyan, M.; Trchounian, A. Antibacterial Effects of Iron Oxide (Fe3O4) Nanoparticles: Distinguishing Concentration-Dependent Effects with Different Bacterial Cells Growth and Membrane-Associated Mechanisms. Appl. Microbiol. Biotechnol. 2019, 103, 2773–2782. [Google Scholar] [CrossRef]
- Bhushan, M.; Kumar, Y.; Periyasamy, L.; Viswanath, A.K. Facile Synthesis of Fe/Zn Oxide Nanocomposites and Study of Their Structural, Magnetic, Thermal, Antibacterial and Cytotoxic Properties. Mater. Chem. Phys. 2018, 209, 233–248. [Google Scholar] [CrossRef]
- Liu, L.-P.; Yang, X.-N.; Ye, L.; Xue, D.-D.; Liu, M.; Jia, S.-R.; Hou, Y.; Chu, L.-Q.; Zhong, C. Preparation and Characterization of a Photocatalytic Antibacterial Material: Graphene Oxide/TiO2/Bacterial Cellulose Nanocomposite. Carbohydr. Polym. 2017, 174, 1078–1086. [Google Scholar] [CrossRef]
- Lučić, M.; Milosavljević, N.; Radetić, M.; Šaponjić, Z.; Radoičić, M.; Kalagasidis Krušić, M. The Potential Application of TiO2/Hydrogel Nanocomposite for Removal of Various Textile Azo Dyes. Sep. Purif. Technol. 2014, 122, 206–216. [Google Scholar] [CrossRef]
- Gupta, V.K.; Gupta, B.; Rastogi, A.; Agarwal, S.; Nayak, A. A Comparative Investigation on Adsorption Performances of Mesoporous Activated Carbon Prepared from Waste Rubber Tire and Activated Carbon for a Hazardous Azo Dye—Acid Blue 113. J. Hazard. Mater. 2011, 186, 891–901. [Google Scholar] [CrossRef]
- Byrne, J.A.; Eggins, B.R.; Brown, N.M.D.; McKinney, B.; Rouse, M. Immobilisation of TiO2 Powder for the Treatment of Polluted Water. Appl. Catal. B Environ. 1998, 17, 25–36. [Google Scholar] [CrossRef]
- Yun, J.; Im, J.S.; Oh, A.; Jin, D.-H.; Bae, T.-S.; Lee, Y.-S.; Kim, H.-I. PH-Sensitive Photocatalytic Activities of TiO2/Poly(Vinyl Alcohol)/Poly(Acrylic Acid) Composite Hydrogels. Mater. Sci. Eng. B 2011, 176, 276–281. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.S.; Ichikawa, H. Hydrogels in Pharmaceutical Formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Rattanaruengsrikul, V.; Pimpha, N.; Supaphol, P. Development of Gelatin Hydrogel Pads as Antibacterial Wound Dressings. Macromol. Biosci. 2009, 9, 1004–1015. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Binaymotlagh, R.; Cerra, S.; Haghighi, F.H.; Di Domenico, E.G.; Sivori, F.; Fratoddi, I.; Mignardi, S.; Palocci, C. Preparation of Hydrogel Composites Using a Sustainable Approach for In Situ Silver Nanoparticles Formation. Materials 2023, 16, 2134. [Google Scholar] [CrossRef]
- Binaymotlagh, R.; Chronopoulou, L.; Hajareh Haghighi, F.; Fratoddi, I.; Palocci, C. Peptide-Based Hydrogels: New Materials for Biosensing and Biomedical Applications. Materials 2022, 15, 5871. [Google Scholar] [CrossRef]
- Binaymotlagh, R.; Chronopoulou, L.; Palocci, C. Peptide-Based Hydrogels: Template Materials for Tissue Engineering. J. Funct. Biomater. 2023, 14, 233. [Google Scholar] [CrossRef]
- Panda, J.J.; Chauhan, V.S. Short Peptide Based Self-Assembled Nanostructures: Implications in Drug Delivery and Tissue Engineering. Polym. Chem. 2014, 5, 4418–4436. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for Tissue Engineering: Scaffold Design Variables and Applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef] [PubMed]
- Chelu, M.; Musuc, A.M.; Aricov, L.; Ozon, E.A.; Iosageanu, A.; Stefan, L.M.; Prelipcean, A.-M.; Popa, M.; Moreno, J.C. Antibacterial Aloe vera Based Biocompatible Hydrogel for Use in Dermatological Applications. Int. J. Mol. Sci. 2023, 24, 3893. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Xu, H.; Philbert, M.A.; Kopelman, R. Ultrafine Hydrogel Nanoparticles: Synthetic Approach and Therapeutic Application in Living Cells. Angew. Chemie Int. Ed. 2007, 46, 2224–2227. [Google Scholar] [CrossRef] [PubMed]
- De Giglio, E.; Cometa, S.; Ricci, M.A.; Cafagna, D.; Savino, A.M.; Sabbatini, L.; Orciani, M.; Ceci, E.; Novello, L.; Tantillo, G.M. Ciprofloxacin-Modified Electrosynthesized Hydrogel Coatings to Prevent Titanium-Implant-Associated Infections. Acta Biomater. 2011, 7, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Ripolles-Avila, C.; Martínez Garcia, M.; Hascoët, A.-S.; Rodriguez-Jerez, J.J. Bactericidal Efficacy of UV Activated TiO2 Nanoparticles against Gram-Positive and Gram-Negative Bacteria on Suspension. CyTA J. Food 2019, 17, 408. [Google Scholar] [CrossRef]
- Schutte-Smith, M.; Erasmus, E.; Mogale, R.; Marogoa, N.; Jayiya, A.; Visser, H.G. Using visible light to activate antiviral and antimicrobial properties of TiO2 nanoparticles in paints and coatings: Focus on new developments for frequent-touch surfaces in hospitals. J. Coat. Technol. Res. 2023, 20, 789–817. [Google Scholar] [CrossRef]
- Xu, Y.; Wei, M.-T.; Ou-Yang, H.D.; Walker, S.G.; Wang, H.Z.; Gordon, C.R.; Guterman, S.; Zawacki, E.; Applebaum, E.; Brink, P.R. Exposure to TiO2 Nanoparticles Increases Staphylococcus aureus Infection of HeLa Cells. J. Nanobiotechnol. 2016, 14, 34. [Google Scholar] [CrossRef]
- Hajareh Haghighi, F.; Binaymotlagh, R.; Chronopoulou, L.; Cerra, S.; Marrani, A.G.; Amato, F.; Palocci, C.; Fratoddi, I. Self-Assembling Peptide-Based Magnetogels for the Removal of Heavy Metals from Water. Gels 2023, 9, 621. [Google Scholar] [CrossRef]
- Hurley, N.; Li, L.; Koenigsmann, C.; Wong, S.S. Surfactant-Free Synthesis of Three-Dimensional Perovskite Titania-Based Micron-Scale Motifs Used as Catalytic Supports for the Methanol Oxidation Reaction. Molecules 2021, 26, 909. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Oliva, A.; Guembe, M. The Current Knowledge on the Pathogenesis of Tissue and Medical Device-Related Biofilm Infections. Microorganisms 2022, 10, 1259. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Jayaramudu, T.; Núñez, D.; Jara, N.; Opazo-Capurro, A.; Varaprasad, K.; Kim, K.; Yallapu, M.M.; Sadiku, R. Hybrid Nanomaterial Composed of Chitosan, Curcumin, ZnO and TiO2 for Antibacterial Therapies. Int. J. Biol. Macromol. 2023, 242, 124814. [Google Scholar] [CrossRef]
- Yang, F.; Liu, S.-L.; Xu, Y.; Walker, S.G.; Cho, W.; Mironava, T.; Rafailovich, M. The Impact of TiO2 Nanoparticle Exposure on Transmembrane Cholesterol Transport and Enhanced Bacterial Infectivity in HeLa Cells. Acta Biomater. 2021, 135, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Dreno, B.; Alexis, A.; Chuberre, B.; Marinovich, M. Safety of Titanium Dioxide Nanoparticles in Cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Azizi-Lalabadi, M.; Ehsani, A.; Divband, B.; Alizadeh-Sani, M. Antimicrobial Activity of Titanium Dioxide and Zinc Oxide Nanoparticles Supported in 4A Zeolite and Evaluation the Morphological Characteristic. Sci. Rep. 2019, 9, 17439. [Google Scholar] [CrossRef]
- Shang, C.; Bu, J.; Song, C. Preparation, Antimicrobial Properties under Different Light Sources, Mechanisms and Applications of TiO2: A Review. Materials 2022, 15, 5820. [Google Scholar] [CrossRef] [PubMed]
- Binaymotlagh, R.; Del Giudice, A.; Mignardi, S.; Amato, F.; Marrani, A.G.; Sivori, F.; Cavallo, I.; Di Domenico, E.G.; Palocci, C.; Chronopoulou, L. Green In Situ Synthesis of Silver Nanoparticles-Peptide Hydrogel Composites: Investigation of Their Antibacterial Activities. Gels 2022, 8, 700. [Google Scholar] [CrossRef] [PubMed]
- Available online: www.sasview.org (accessed on 29 November 2023).
- Svergun, D.I. Determination of the Regularization Parameter in Indirect-Transform Methods Using Perceptual Criteria. J. Appl. Crystallogr. 1992, 25, 495–503. [Google Scholar] [CrossRef]
- Cavallo, I.; Sivori, F.; Truglio, M.; De Maio, F.; Lucantoni, F.; Cardinali, G.; Pontone, M.; Bernardi, T.; Sanguinetti, M.; Capitanio, B.; et al. Skin Dysbiosis and Cutibacterium Acnes Biofilm in Inflammatory Acne Lesions of Adolescents. Sci. Rep. 2022, 12, 21104. [Google Scholar] [CrossRef] [PubMed]
- Sivori, F.; Cavallo, I.; Kovacs, D.; Guembe, M.; Sperduti, I.; Truglio, M.; Pasqua, M.; Prignano, G.; Mastrofrancesco, A.; Toma, L.; et al. Role of Extracellular DNA in Dalbavancin Activity against Methicillin-Resistant Staphylococcus aureus (MRSA) Biofilms in Patients with Skin and Soft Tissue Infections. Microbiol. Spectr. 2022, 10, e0035122. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binaymotlagh, R.; Hajareh Haghighi, F.; Di Domenico, E.G.; Sivori, F.; Truglio, M.; Del Giudice, A.; Fratoddi, I.; Chronopoulou, L.; Palocci, C. Biosynthesis of Peptide Hydrogel–Titania Nanoparticle Composites with Antibacterial Properties. Gels 2023, 9, 940. https://doi.org/10.3390/gels9120940
Binaymotlagh R, Hajareh Haghighi F, Di Domenico EG, Sivori F, Truglio M, Del Giudice A, Fratoddi I, Chronopoulou L, Palocci C. Biosynthesis of Peptide Hydrogel–Titania Nanoparticle Composites with Antibacterial Properties. Gels. 2023; 9(12):940. https://doi.org/10.3390/gels9120940
Chicago/Turabian StyleBinaymotlagh, Roya, Farid Hajareh Haghighi, Enea Gino Di Domenico, Francesca Sivori, Mauro Truglio, Alessandra Del Giudice, Ilaria Fratoddi, Laura Chronopoulou, and Cleofe Palocci. 2023. "Biosynthesis of Peptide Hydrogel–Titania Nanoparticle Composites with Antibacterial Properties" Gels 9, no. 12: 940. https://doi.org/10.3390/gels9120940