Fabrication and Characterisation of Hydrogels with Reversible Wrinkled Surfaces for Limbal Study and Reconstruction
Abstract
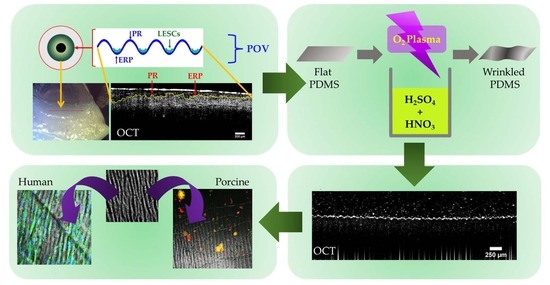
:1. Introduction
2. Results and Discussion
2.1. Stretching Frame for Creating a reversible Wrinkle Pattern on PDMS Substrates
2.2. Imaging of the Wrinkled Substrates with Scanning Electron Microscopy
2.3. Mechanical Properties of the Wrinkled Substrates
2.4. Depth Analysis of the Dual-Treated Substrates
2.5. OCT of the Dynamization of Dual-Treated Substrates
2.6. Human Primary Limbal Cells Seeded on Static Wrinkled Substrates
2.7. Porcine Limbal Epithelial Stem Cells on Static Substrates
2.8. Cellular Response to the Reversible Wrinkled Substrate
3. Conclusions
4. Materials and Methods
4.1. Preparation of PDMS Substrate
4.2. Preparation of the PDMS Substrate with a Reversible Winkle Pattern
4.3. Gelatin Methacrylate (GelMa) Production and Coating
4.4. Optical Coherence Tomography
4.5. Scanning Electron Microscopy
4.6. Tensile Testing
4.7. Cell Extraction and Culture
4.8. Immunofluorescence and Imaging
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mei, N.; Wu, Y.; Chen, B.; Zhuang, T.; Yu, X.; Sui, B.; Ding, T.; Liu, X. 3D-printed mesoporous bioactive glass/GelMA biomimetic scaffolds for osteogenic/cementogenic differentiation of periodontal ligament cells. Front. Bioeng. Biotechnol. 2022, 10, 950970. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.; Yu, J.; Chen, X.; Wang, R.; Zhang, M.; Zhang, Q.; Zhang, Y.; Wang, S.; Cheng, Y. Bioactive skin-mimicking hydrogel band-aids for diabetic wound healing and infectious skin incision treatment. Bioact. Mater. 2021, 6, 3962–3975. [Google Scholar] [CrossRef]
- Jackson, N.D.; Nyska, A.; Palmanovich, E.; Nyska, M. The biointegration profile of fiber-reinforced plates following tibial im-plantation in sheep. J. Orthop. Res. 2023. [Google Scholar] [CrossRef]
- Gouveia, R.M.; Vajda, F.; Wibowo, J.A.; Figueiredo, F.; Connon, C.J. YAP, ΔNp63, and β-Catenin Signaling Pathways Are In-volved in the Modulation of Corneal Epithelial Stem Cell Phenotype Induced by Substrate Stiffness. Cells 2019, 8, 347. [Google Scholar] [CrossRef]
- Burdick, J.A.; Vunjak-Novakovic, G. Engineered Microenvironments for Controlled Stem Cell Differentiation. Tissue Eng. Part A 2009, 15, 205–219. [Google Scholar] [CrossRef]
- Mahadik, B.P.; Haba, S.P.; Skertich, L.J.; Harley, B.A. The use of covalently immobilized stem cell factor to selectively affect hematopoietic stem cell activity within a gelatin hydrogel. Biomaterials 2015, 67, 297–307. [Google Scholar] [CrossRef]
- Pires, F.; Ferreira, Q.; Rodrigues, C.A.V.; Morgado, J.; Ferreira, F.C. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: Expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochim. Biophys. Acta 2015, 1850, 1158–1168. [Google Scholar] [CrossRef]
- Rödling, L.; Schwedhelm, I.; Kraus, S.; Bieback, K.; Hansmann, J.; Lee-Thedieck, C. 3D models of the hematopoietic stem cell niche under steady-state and active conditions. Sci. Rep. 2017, 7, 4625. [Google Scholar] [CrossRef]
- Seo, J.; Shin, J.-Y.; Leijten, J.; Jeon, O.; Öztürk, A.B.; Rouwkema, J.; Li, Y.; Shin, S.R.; Hajiali, H.; Alsberg, E.; et al. Interconnectable Dynamic Compression Bioreactors for Combinatorial Screening of Cell Mechanobiology in Three Dimensions. ACS Appl. Mater. Interfaces 2018, 10, 13293–13303. [Google Scholar] [CrossRef]
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract. Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef]
- Thoft, R.A.; Friend, J. The X, Y, Z hypothesis of corneal epithelial maintenance. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1442–1443. [Google Scholar]
- Vemuganti, G.K.; Fatima, A.; Madhira, S.L.; Basti, S.; Sangwan, V.S. Chapter 5 Limbal Stem Cells. Application in Ocular Bio-medicine. Int. Rev. Cell Mol. Biol. 2009, 275, 133–181. [Google Scholar]
- A Shanmuganathan, V.; Foster, T.; Kulkarni, B.B.; Hopkinson, A.; Gray, T.; Powe, D.G.; Lowe, J.; Dua, H.S. Morphological characteristics of the limbal epithelial crypt. Br. J. Ophthalmol. 2007, 91, 514–519. [Google Scholar] [CrossRef]
- Lagali, N.; Edén, U.; Utheim, T.P.; Chen, X.; Riise, R.; Dellby, A.; Fagerholm, P. In vivo morphology of the limbal palisades of vogt correlates with progressive stem cell deficiency in aniridia-related keratopathy. Investig. Opthalmology Vis. Sci. 2013, 54, 5333–5342. [Google Scholar] [CrossRef]
- Grieve, K.; Ghoubay, D.; Georgeon, C.; Thouvenin, O.; Bouheraoua, N.; Paques, M.; Borderie, V.M. Three-dimensional structure of the mammalian limbal stem cell niche. Exp. Eye Res. 2015, 140, 75–84. [Google Scholar] [CrossRef]
- Li, P.; An, L.; Reif, R.; Shen, T.T.; Johnstone, M.; Wang, R.K. In vivo microstructural and microvascular imaging of the human corneo-scleral limbus using optical coherence tomography. Biomed. Opt. Express 2011, 2, 3109–3118. [Google Scholar] [CrossRef]
- Lathrop, K.L.; Gupta, D.; Kagemann, L.; Schuman, J.S.; SundarRaj, N. Optical coherence tomography as a Rapid, Accurate, noncontact method of visualizing the palisades of vogt. Investig. Opthalmology Vis. Sci. 2012, 53, 1381–1387. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Mukherjee, A.; Pisano, S.; Dimri, S.; Knaane, E.; Altshuler, A.; Nasser, W.; Dey, S.; Shi, L.; Mizrahi, I.; et al. The biophysical property of the limbal niche maintains stemness through YAP. Cell Death Differ. 2023, 30, 1601–1614. [Google Scholar] [CrossRef]
- Xu, X.; Ma, L.; Wu, Y.; Tang, L. Micropillar-based culture platform induces epithelial–mesenchymal transition in the alveolar epithelial cell line. J. Biomed. Mater. Res. Part A 2018, 106, 3165–3174. [Google Scholar] [CrossRef]
- Kyle, D.J.T.; Oikonomou, A.; Hill, E.; Bayat, A. Development and functional evaluation of biomimetic silicone surfaces with hierarchical micro/nano-topographical features demonstrates favourable in vitro foreign body response of breast-derived fibroblasts. Biomaterials 2015, 52, 88–102. [Google Scholar] [CrossRef]
- Dimmock, R.L.; Wang, X.; Fu, Y.; El Haj, A.J.; Yang, Y. Biomedical Applications of Wrinkling Polymers. Recent Prog. Mater. 2020, 2, 1–31. [Google Scholar] [CrossRef]
- Levis, H.J.; Massie, I.; Dziasko, M.A.; Kaasi, A.; Daniels, J.T. Rapid tissue engineering of biomimetic human corneal limbal crypts with 3D niche architecture. Biomaterials 2013, 34, 8860–8868. [Google Scholar] [CrossRef]
- Polisetti, N.; Sorokin, L.; Okumura, N.; Koizumi, N.; Kinoshita, S.; Kruse, F.E.; Schlötzer-Schrehardt, U. Laminin-511 and -521-based matrices for efficient ex vivo-expansion of human limbal epithelial progenitor cells. Sci. Rep. 2017, 7, 5152. [Google Scholar] [CrossRef]
- Asejczyk-Widlicka, M.; Śródka, D.W.; Kasprzak, H.; Pierscionek, B.K. Modelling the elastic properties of the anterior eye and their contribution to maintenance of image quality: The role of the limbus. Eye 2007, 21, 1087–1094. [Google Scholar] [CrossRef]
- Tang, Q.; Li, X.; Lai, C.; Li, L.; Wu, H.; Wang, Y.; Shi, X. Fabrication of a hydroxyapatite-PDMS microfluidic chip for bone-related cell culture and drug screening. Bioact. Mater. 2021, 6, 169–178. [Google Scholar] [CrossRef]
- Si, J.; Cui, Z.; Xie, P.; Song, L.; Wang, Q.; Liu, Q.; Liu, C. Characterization of 3D elastic porous polydimethylsiloxane (PDMS) cell scaffolds fabricated by VARTM and particle leaching. J. Appl. Polym. Sci. 2016, 133, 42909. [Google Scholar] [CrossRef]
- Wang, Z.; Volinsky, A.A.; Gallant, N.D. Crosslinking effect on polydimethylsiloxane elastic modulus measured by custom-built compression instrument. J. Appl. Polym. Sci. 2014, 131, 41050. [Google Scholar] [CrossRef]
- Tong, L.; Zhou, W.; Zhao, Y.; Yu, X.; Wang, H.; Chu, P.K. Enhanced cytocompatibility and reduced genotoxicity of polydime-thylsiloxane modified by plasma immersion ion implantation. Colloids Surf. B Biointerfaces 2016, 148, 139–146. [Google Scholar] [CrossRef]
- Sharma, D.; Jia, W.; Long, F.; Pati, S.; Chen, Q.; Qyang, Y.; Lee, B.; Choi, C.K.; Zhao, F. Polydopamine and collagen coated micro-grated polydimethylsiloxane for human mesenchymal stem cell culture. Bioact. Mater. 2019, 4, 142–150. [Google Scholar] [CrossRef]
- Gehlen, D.B.; De Lencastre Novaes, L.C.; Long, W.; Ruff, A.J.; Jakob, F.; Haraszti, T.; Chandorkar, Y.; Yang, L.; van Rijn, P.; Schwaneberg, U.; et al. Rapid and Robust Coating Method to Render Polydimethylsiloxane Surfaces Cell-Adhesive. ACS Appl. Mater. Interfaces. 2019, 11, 41091–41099. [Google Scholar] [CrossRef]
- Watanabe, M.; Hashimoto, R. Area-selective microwrinkle formation on poly(dimethylsiloxane) by treatment with strong acid. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 167–174. [Google Scholar] [CrossRef]
- Glatz, B.A.; Fery, A. The influence of plasma treatment on the elasticity of the in situ oxidized gradient layer in PDMS: Towards crack-free wrinkling. Soft Matter 2018, 15, 65–72. [Google Scholar] [CrossRef]
- Patel, D.V.; Sherwin, T.; McGhee, C.N.J. Laser Scanning In Vivo Confocal Microscopy of the Normal Human Corneoscleral Limbus. Investig. Opthalmology Vis. Sci. 2006, 47, 2823–2827. [Google Scholar] [CrossRef]
- Haagdorens, M.; Cėpla, V.; Melsbach, E.; Koivusalo, L.; Skottman, H.; Griffith, M.; Valiokas, R.; Zakaria, N.; Pintelon, I.; Tassignon, M.-J. In Vitro Cultivation of Limbal Epithelial Stem Cells on Surface-Modified Crosslinked Collagen Scaffolds. Stem Cells Int. 2019, 2019, 7867613. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimmock, R.L.; Rotherham, M.; El Haj, A.J.; Yang, Y. Fabrication and Characterisation of Hydrogels with Reversible Wrinkled Surfaces for Limbal Study and Reconstruction. Gels 2023, 9, 915. https://doi.org/10.3390/gels9110915
Dimmock RL, Rotherham M, El Haj AJ, Yang Y. Fabrication and Characterisation of Hydrogels with Reversible Wrinkled Surfaces for Limbal Study and Reconstruction. Gels. 2023; 9(11):915. https://doi.org/10.3390/gels9110915
Chicago/Turabian StyleDimmock, Ryan L., Michael Rotherham, Alicia J. El Haj, and Ying Yang. 2023. "Fabrication and Characterisation of Hydrogels with Reversible Wrinkled Surfaces for Limbal Study and Reconstruction" Gels 9, no. 11: 915. https://doi.org/10.3390/gels9110915