Application of Taro (Colocasia esculenta) Mucilage as a Promising Antimicrobial Agent to Extend the Shelf Life of Fresh-Cut Brinjals (Eggplants)
Abstract
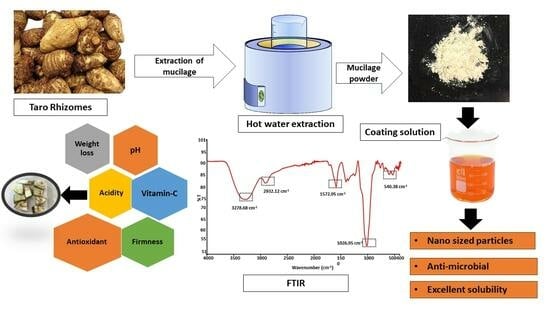
:1. Introduction
2. Results and Discussion
2.1. Mucilage Yield, Zeta Potential, and Particle Size
2.2. Rheological Behavior of the Coating Gel Solution
2.3. Determination of Functional Groups
2.4. Scanning Electron Microscopy (SEM) of TMCG
2.5. Physicochemical Properties of Edible Coated Cut Brinjals
2.6. Microbial Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation
4.3. Extraction of TM
4.4. Formulations of Edible Coating Gel Solutions
4.5. Characterization of Coating Gel Solution
4.5.1. Particle Size and Zeta Potential
4.5.2. Rheological Behavior
4.5.3. Determination of Functional Groups
4.5.4. Scanning Electron Microscopy (SEM) of TMCG
4.6. Application of the Coating Gel Solution of Cut Brinjals
4.6.1. Weight Loss and pH Value of Cut Brinjals
4.6.2. TSS and Acidity of Cut Brinjals
4.6.3. Antioxidant Activity of Cut Brinjals
4.6.4. Ascorbic Acid Content of Cut Brinjals
4.6.5. Firmness
4.7. Microbial Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manthei, A.; López-Gámez, G.; Martín-Belloso, O.; Elez-Martínez, P.; Soliva-Fortuny, R. Relationship between Physicochemical, Techno-Functional and Health-Promoting Properties of Fiber-Rich Fruit and Vegetable By-Products and Their Enhancement by Emerging Technologies. Foods 2023, 12, 3720. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.M.; Sameen, A.; Aadil, R.M.; Shahid, M.; Sezen, S.; Zarrabi, A.; Ozdemir, B.; Sevindik, M.; Kaplan, D.N.; Selamoglu, Z.; et al. Citrus genus and its waste utilization: A review on health-promoting activities and industrial application. Evid.-Based Complement. Altern. Med. 2021, 2021, 2488804. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Azadfar, E.; Rusu, A.; Trif, M.; Poushi, M.K.; Wang, Y. Improving the Shelf Life of Peeled Fresh Almond Kernels by Edible Coating with Mastic Gum. Coatings 2021, 11, 618. [Google Scholar] [CrossRef]
- Ghag, S.S.; Gokhale, J.S.; Lele, S.S. Effect of chemical pretreatment on quality attributes of the cashew apple. J. Food Sci. 2023, 88, 2353–2367. [Google Scholar] [CrossRef]
- Lyn, F.H.; Adilah, Z.M.; Nor-Khaizura, M.A.R.; Jamilah, B.; Hanani, Z.N. Application of modified atmosphere and active packaging for oyster mushroom (Pleurotus ostreatus). Food Packag. Shelf Life 2020, 23, 100451. [Google Scholar] [CrossRef]
- Kalpana, S.; Priyadarshini, S.R.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Intelligent packaging: Trends and applications in food systems. Trends Food Sci. Technol. 2019, 93, 145–157. [Google Scholar] [CrossRef]
- Fiore, A.; Park, S.; Volpe, S.; Torrieri, E.; Masi, P. Active packaging based on PLA and chitosan-caseinate enriched rosemary essential oil coating for fresh minced chicken breast application. Food Packag. Shelf Life 2021, 29, 100708. [Google Scholar] [CrossRef]
- Goksen, G.; Demir, D.; Dhama, K.; Kumar, M.; Shao, P.; Xie, F.; Echegaray, N.; Lorenzo, J.M. Mucilage polysaccharide as a plant secretion: Potential trends in food and biomedical applications. Int. J. Biol. Macromol. 2023, 230, 123146. [Google Scholar] [CrossRef]
- Kaith, B.S.; Singh, A.; Sud, D. A new horizon for structural modifications and comprehensive applications of Colocasia esculenta (L.). Schott. Mater. Today Proc. 2022, 53, 269–272. [Google Scholar] [CrossRef]
- Hendek Ertop, M.; Atasoy, R.; Akın, Ş.S. Evaluation of taro [Colocasia Esculenta (L.) Schott] flour as a hydrocolloid on the physicochemical, rheological, and sensorial properties of milk pudding. J. Food Process. Preserv. 2019, 43, e14103. [Google Scholar] [CrossRef]
- Tosif, M.M.; Najda, A.; Klepacka, J.; Bains, A.; Chawla, P.; Kumar, A.; Sharma, M.; Sridhar, K.; Gautam, S.P.; Kaushik, R. A concise review on taro mucilage: Extraction techniques, chemical composition, characterization, applications, and health attributes. Polymers 2022, 14, 1163. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Ahmed, T.; Rana, M.R.; Hoque, M.M.; Ahmed, M.F.; Sharma, M.; Sridhar, K.; Ara, R.; Stephen Inbaraj, B. Fabrication and Characterization of ZnO Nanoparticles-Based Biocomposite Films Prepared Using Carboxymethyl Cellulose, Taro Mucilage, and Black Cumin Seed Oil for Evaluation of Antioxidant and Antimicrobial Activities. Agronomy 2023, 13, 147. [Google Scholar] [CrossRef]
- Farousha, K.; Rangaraj, V.M.; Rambabu, K.; Haija, M.A.; Banat, F. Development of date seed extract encapsulated MCM-41: Characterization, release kinetics, antioxidant and antibacterial studies. Food Biosci. 2023, 53, 102563. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Hai, A.; Banat, F. Subcritical Water Extraction of Mango Seed Kernels and Its Application for Cow Ghee Preservation. Processes 2023, 11, 1379. [Google Scholar] [CrossRef]
- Safdar, B.; Pang, Z.; Liu, X.; Jatoi, M.A.; Rashid, M.T. Rheological and tribological nature of flaxseed gum influenced by concentration and temperature and its application as a coating agent for potato chips. J. Food Sci. 2022, 87, 2058–2071. [Google Scholar] [CrossRef]
- Andrade, L.A.; de Oliveira Silva, D.A.; Nunes, C.A.; Pereira, J. Experimental techniques for the extraction of taro mucilage with enhanced emulsifier properties using chemical characterization. Food Chem. 2020, 327, 127095. [Google Scholar] [CrossRef]
- Thakur, S.; Bains, A.; Sridhar, K.; Kaushik, R.; Gupta, V.K.; Chawla, P.; Sharma, M. Gum Arabic/guar gum based biopolymeric nanohydrogel for shelf-life enhancement of grapes and photocatalytic dye reduction. Ind. Crops Prod. 2023, 203, 117114. [Google Scholar] [CrossRef]
- Tosif, M.M.; Bains, A.; Dhull, S.B.; Chawla, P.; Goksen, G. Effect of Aloe vera and carboxymethyl cellulose-derived binary blend edible coating on the shelf life of fresh-cut apple. Food Sci. Nutr. 2023, 11, 6987–6999. [Google Scholar] [CrossRef]
- Amanullah, S.; Jahangir, M.M.; Ikram, R.M.; Sajid, M.; Abbas, F.; Mallano, A.I. Aloe vera coating efficiency on shelf life of eggplants at differential storage temperatures. J. Northeast. Agric. Univ. (Engl. Ed.) 2016, 23, 15–25. [Google Scholar] [CrossRef]
- Anjum, M.A.; Akram, H.; Zaidi, M.; Ali, S. Effect of gum arabic and Aloe vera gel based edible coatings in combination with plant extracts on postharvest quality and storability of ‘Gola’guava fruits. Sci. Hortic. 2020, 271, 109506. [Google Scholar] [CrossRef]
- Singh, S.; Singh, B.; Alam, T. Evaluation of shelf-life, antioxidant activity and nutritional quality attributes in carnauba wax coated eggplant genotypes. J. Food Sci. Technol. 2019, 56, 4826–4833. [Google Scholar] [CrossRef] [PubMed]
- Rangaraj, V.M.; Devaraju, S.; Rambabu, K.; Banat, F.; Mittal, V. Silver-sepiolite (Ag–Sep) hybrid reinforced active gelatin/date waste extract (DSWE) blend composite films for food packaging application. Food Chem. 2022, 369, 130983. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef]
- Miteluț, A.C.; Popa, E.E.; Drăghici, M.C.; Popescu, P.A.; Popa, V.I.; Bujor, O.C.; Ion, V.A.; Popa, M.E. Latest developments in edible coatings on minimally processed fruits and vegetables: A review. Foods 2021, 10, 2821. [Google Scholar] [CrossRef]
- Hassoun, A.; Cropotova, J.; Trif, M.; Rusu, A.V.; Bobiş, O.; Nayik, G.A.; Jagdale, Y.D.; Saeed, F.; Afzaal, M.; Mostashari, P.; et al. Consumer acceptance of new food trends resulting from the fourth industrial revolution technologies: A narrative review of literature and future perspectives. Front. Nutr. 2022, 9, 972154. [Google Scholar] [CrossRef]
- Iqbal, M.W.; Riaz, T.; Yasmin, I.; Leghari, A.A.; Amin, S.; Bilal, M.; Qi, X. Chitosan-based materials as edible coating of cheese: A review. Starch-Stärke 2021, 73, 2100088. [Google Scholar] [CrossRef]
- Shanta, S.S.; Ahmed, T.; Jubayer, M.F.; Sharma, M.; Sridhar, K.; Hoque, M.M.; Rana, M.R.; Inbaraj, B.S. Effect of Taro Corm Mucilage and Black Seed Oil as Edible Coatings on the Shelf-Life and Quality of Fresh Guava. Agronomy 2023, 13, 538. [Google Scholar] [CrossRef]
- Moreira, B.R.; Pereira-Junior, M.A.; Fernandes, K.F.; Batista, K.A. An ecofriendly edible coating using cashew gum polysaccharide and polyvinyl alcohol. Food Biosci. 2020, 37, 100722. [Google Scholar] [CrossRef]
- Le, K.H.; Nguyen, M.D.B.; Dai Tran, L.; Thi, H.P.N.; Van Tran, C.; Van Tran, K.; Thi, H.P.N.; Thi, N.D.; Yoon, Y.S.; Nguyen, D.D.; et al. A novel antimicrobial ZnO nanoparticles-added polysaccharide edible coating for the preservation of postharvest avocado under ambient conditions. Prog. Org. Coat. 2021, 158, 106339. [Google Scholar] [CrossRef]
- Panahirad, S.; Naghshiband-Hassani, R.; Bergin, S.; Katam, R.; Mahna, N. Improvement of postharvest quality of plum (Prunus domestica L.) using polysaccharide-based edible coatings. Plants 2020, 9, 1148. [Google Scholar] [CrossRef]
- Abbasi, A.; Sabahi, S.; Bazzaz, S.; Tajani, A.G.; Lahouty, M.; Aslani, R.; Hosseini, H. An edible coating utilizing Malva sylvestris seed polysaccharide mucilage and postbiotic from Saccharomyces cerevisiae var. boulardii for the preservation of lamb meat. Int. J. Biol. Macromol. 2023, 246, 125660. [Google Scholar] [CrossRef]
- de Souza, E.L.; Lundgren, G.A.; de Oliveira, K.Á.; Berger, L.R.; Magnani, M. An analysis of the published literature on the effects of edible coatings formed by polysaccharides and essential oils on postharvest microbial control and overall quality of fruit. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1947–1967. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.S.; Anjum, M.A.; Naz, S.; Ali, S.; Hussain, S.; Azam, M.; Sardar, H.; Khaliq, G.; Canan, İ.; Ejaz, S. Incorporation of ascorbic acid in chitosan-based edible coating improves postharvest quality and storability of strawberry fruits. Int. J. Biol. Macromol. 2021, 189, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Ding, J.; Liu, C.; Li, H.; Wang, C.; Lin, Y.; Sameen, D.E.; Hossen, M.A.; Chen, M.; Yan, J.; et al. Konjac glucomannan/low-acyl gellan gum edible coating containing thymol microcapsule regulates cell wall polysaccharides disassembly and delays postharvest softening of blueberries. Postharvest Biol. Technol. 2023, 204, 112449. [Google Scholar] [CrossRef]
- Paolucci, M.; Di Stasio, M.; Sorrentino, A.; La Cara, F.; Volpe, M.G. Active edible polysaccharide-based coating for preservation of fresh figs (Ficus carica L.). Foods 2020, 9, 1793. [Google Scholar] [CrossRef]








| Treatment | Concentration (%) | Average Particle Size (nm) | Zeta Potential (mV) |
|---|---|---|---|
| T1 | 1 | 224 ± 0.68 | −24.18 ± 0.44 |
| T2 | 2 | 204 ± 0.94 | −16.14 ± 0.67 |
| T3 | 3 | 189 ± 0.73 | −31.87 ± 0.35 |
| T4 | 4 | 171 ± 0.55 | −11.27 ± 0.27 |
| T5 | 5 | 154 ± 0.81 | −27.22 ± 0.75 |
| T6 | 6 | 166 ± 1.68 | −36.46 ± 0.34 |
| T7 | 7 | 187 ± 0.47 | −20.67 ± 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tosif, M.M.; Bains, A.; Goksen, G.; Ali, N.; Rusu, A.V.; Trif, M.; Chawla, P. Application of Taro (Colocasia esculenta) Mucilage as a Promising Antimicrobial Agent to Extend the Shelf Life of Fresh-Cut Brinjals (Eggplants). Gels 2023, 9, 904. https://doi.org/10.3390/gels9110904
Tosif MM, Bains A, Goksen G, Ali N, Rusu AV, Trif M, Chawla P. Application of Taro (Colocasia esculenta) Mucilage as a Promising Antimicrobial Agent to Extend the Shelf Life of Fresh-Cut Brinjals (Eggplants). Gels. 2023; 9(11):904. https://doi.org/10.3390/gels9110904
Chicago/Turabian StyleTosif, Mansuri M., Aarti Bains, Gulden Goksen, Nemat Ali, Alexandru Vasile Rusu, Monica Trif, and Prince Chawla. 2023. "Application of Taro (Colocasia esculenta) Mucilage as a Promising Antimicrobial Agent to Extend the Shelf Life of Fresh-Cut Brinjals (Eggplants)" Gels 9, no. 11: 904. https://doi.org/10.3390/gels9110904