The Sustainable Bioactive Dyeing of Textiles: A Novel Strategy Using Bacterial Pigments, Natural Antibacterial Ingredients, and Deep Eutectic Solvents
Abstract
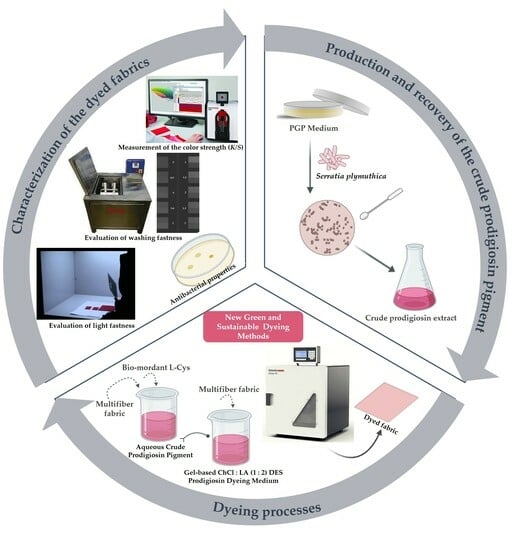
:1. Introduction
2. Results and Discussion
2.1. Evaluation of the Color Strength and Equalization of the Dyed Fabrics
2.1.1. The Effect of Temperature
2.1.2. The Effect of the Dyeing Aids Addition
The Addition of Salts
The Addition of Mordants
2.1.3. The Effect of the Use of Gel-Based Deep Eutectic Solvents (DESs) as a Prodigiosin Dyeing Medium
2.2. Analysis of the Surface Morphology of Dyed Fabrics through SEM
2.3. Evaluation of Fastness Properties of Dyed Fabrics
2.3.1. Assessment of the Washing Fastness Properties
2.3.2. Assessment of the Light Fastness Properties
2.4. Evaluation of the Antibacterial Activity of Dyed Fabrics
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Production and Recovery of Prodigiosin Pigment
4.3. Preparation of the Crude Gel Prodigiosin Pigment Solution and Dyeing Process
Optimization of Dyeing Conditions
- −
- The addition of salts: salts that are often used for dyeing textile fibers, like NaOH (12 mL/L) and Na2S2O4 (4 g/L), were added to the aqueous crude gel prodigiosin pigment bath in order to evaluate the effectiveness of these salts in the dyeing process.
- −
- The addition of mordants: The mordant process was performed based on the simultaneous mordanting methodology using two different mordants: FeSO4, a metallic mordant, and L-Cys, an amino acid bio-mordant, at 1.0%, 3.0%, and 5.0% owf under the same dyeing conditions. Moreover, the effect of the crude gel prodigiosin pigment bath’s pH on dyeing the multifiber fabrics was evaluated at pH = 4.0 and pH = 8.3 in order to achieve an acidic pH during dyeing with the FeSO4 metallic mordant and an alkaline pKa of the bio-mordant L-Cys, respectively.
4.4. Characterization of the Dyed Fabrics
4.4.1. Color Strength Measurements of Dyed Fabrics
4.4.2. Analysis of the Surface Morphology of Dyed Fabrics through SEM
4.4.3. Evaluation of Fastness Properties of Dyed Fabrics
4.4.4. Evaluation of the Antibacterial Activity of Dyed Fabrics
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Haji, A.; Naebe, M. Cleaner Dyeing of Textiles Using Plasma Treatment and Natural Dyes: A Review. J. Clean. Prod. 2020, 265, 121866. [Google Scholar] [CrossRef]
- Kramar, A.; Kostic, M.M. Bacterial Secondary Metabolites as Biopigments for Textile Dyeing. Textiles 2022, 2, 252–264. [Google Scholar] [CrossRef]
- Metwally, R.A.; El Sikaily, A.; El-Sersy, N.A.; Ghozlan, H.A.; Sabry, S.A. Antimicrobial Activity of Textile Fabrics Dyed with Prodigiosin Pigment Extracted from Marine Serratia rubidaea RAM_Alex Bacteria. Egypt. J. Aquat. Res. 2021, 47, 301–305. [Google Scholar] [CrossRef]
- Silva, P.M.d.S.; Fiaschitello, T.R.; de Queiroz, R.S.; Freeman, H.S.; da Costa, S.A.; Leo, P.; Montemor, A.F.; da Costa, S.M. Natural Dye from Croton urucurana Baill. Bark: Extraction, Physicochemical Characterization, Textile Dyeing and Color Fastness Properties. Dyes Pigm. 2020, 173, 107953. [Google Scholar] [CrossRef]
- Amutha, K.; Grace Annapoorani, S.; Sudhapriya, N. Dyeing of Textiles with Natural Dyes Extracted from Terminalia arjuna and Thespesia populnea Fruits. Ind. Crops Prod. 2020, 148, 112303. [Google Scholar] [CrossRef]
- Zheng, M.; Sun, Y.; Li, C.; Lu, Y.; Dai, Y.; Wang, Z. A Novel and Eco-Friendly Approach for Dyeing Polyester Fabrics by Liquid Disperse Dyes Treated with Deep Eutectic Solvent. Color. Technol. 2023, 139, 552–564. [Google Scholar] [CrossRef]
- Jiang, Z.; Cui, Y.; Zheng, G.; Wei, Y.; Wang, Q.; Zhou, M.; Wang, P.; Yu, Y. An Innovative, Low-Cost and Environment-Friendly Approach by Using a Deep Eutectic Solvent as the Water Substitute to Minimize Waste in the Textile Industry and for Better Clothing Performance. Green Chem. 2022, 24, 5904–5917. [Google Scholar] [CrossRef]
- Aruldass, C.A.; Aziz, A.; Venil, C.K.; Khasim, A.R.; Ahmad, W.A. Utilization of Agro-Industrial Waste for the Production of Yellowish-Orange Pigment from Chryseobacterium artocarpi CECT 8497. Int. Biodeterior. Biodegrad. 2016, 113, 342–349. [Google Scholar] [CrossRef]
- Panesar, R.; Kaur, S.; Panesar, P.S. Production of Microbial Pigments Utilizing Agro-Industrial Waste: A Review. Curr. Opin. Food Sci. 2015, 1, 70–76. [Google Scholar] [CrossRef]
- Venil, C.K.; Devi, P.R.; Ahmad, W.A. Agro-Industrial Waste as Substrates for the Production of Bacterial Pigment. In Valorisation of Agro-Industrial Residues—Volume I: Biological Approaches; Springer: Cham, Switzerland, 2020; pp. 149–162. [Google Scholar]
- Amorim, L.F.A.; Fangueiro, R.; Gouveia, I.C. Characterization of Bioactive Colored Materials Produced from Bacterial Cellulose and Bacterial Pigments. Materials 2022, 15, 2069. [Google Scholar] [CrossRef] [PubMed]
- Amorim, L.F.A.; Mouro, C.; Riool, M.; Gouveia, I.C. Antimicrobial Food Packaging Based on Prodigiosin-Incorporated Double-Layered Bacterial Cellulose and Chitosan Composites. Polymers 2022, 14, 315. [Google Scholar] [CrossRef]
- Arivizhivendhan, K.V.; Mahesh, M.; Boopathy, R.; Swarnalatha, S.; Regina Mary, R.; Sekaran, G. Antioxidant and Antimicrobial Activity of Bioactive Prodigiosin Produces from Serratia marcescens Using Agricultural Waste as a Substrate. J. Food Sci. Technol. 2018, 55, 2661–2670. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, A.; Padalia, U. Optimization of Prodigiosin Biosynthesis by Serratia marcescens Using Unconventional Bioresources. J. Genet. Eng. Biotechnol. 2020, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Elkenawy, N.M.; Yassin, A.S.; Elhifnawy, H.N.; Amin, M.A. Optimization of Prodigiosin Production by Serratia marcescens Using Crude Glycerol and Enhancing Production Using Gamma Radiation. Biotechnol. Rep. 2017, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Lazic, J.; Skaro Bogojevic, S.; Vojnovic, S.; Aleksic, I.; Milivojevic, D.; Kretzschmar, M.; Gulder, T.; Petkovic, M.; Nikodinovic-Runic, J. Synthesis, Anticancer Potential and Comprehensive Toxicity Studies of Novel Brominated Derivatives of Bacterial Biopigment Prodigiosin from Serratia marcescens ATCC 27117. Molecules 2022, 27, 3729. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.L.T.; Nguyen, T.C.; Do, T.T.; Vu, T.L.; Nguyen, T.T.; Do, T.T.; Nguyen, T.H.T.; Le, T.H.; Trinh, D.K.; Nguyen, T.A.T. Study on the Anticancer Activity of Prodigiosin from Variants of Serratia marcescens QBN VTCC 910026. Biomed. Res. Int. 2022, 2022, 4053074. [Google Scholar] [CrossRef] [PubMed]
- Picha, P.; Kale, D.; Dave, I.; Pardeshi, S. Comparative Studies on Prodigiosin Production by Serratia marcescens Using Various Crude Fatty Acid Sources-Its Characterization and Applications. Int. J. Curr. Microbiol. Appl. Sci. 2015, 2, 254–267. [Google Scholar]
- Ren, Y.; Gong, J.; Fu, R.; Li, Z.; Li, Q.; Zhang, J.; Yu, Z.; Cheng, X. Dyeing and Antibacterial Properties of Cotton Dyed with Prodigiosins Nanomicelles Produced by Microbial Fermentation. Dyes Pigm. 2017, 138, 147–153. [Google Scholar] [CrossRef]
- Venil, C.K.; Dufossé, L.; Velmurugan, P.; Malathi, M.; Lakshmanaperumalsamy, P. Extraction and Application of Pigment from Serratia marcescens SB08, an Insect Enteric Gut Bacterium, for Textile Dyeing. Textiles 2021, 1, 21–36. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheng, Q.; Shen, Z.; Fan, B.; Xu, Y.; Cao, Y.; Peng, F.; Zhao, J.; Xue, B. Structure of Prodigiosin from Serratia marcescens NJZT-1 and Its Cytotoxicity on TSC2-Null Cells. Food Sci. Technol. 2020, 41, 189–196. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Wang, S.L.; Nguyen, V.B. Recent Advances in Eco-Friendly and Scaling-Up Bioproduction of Prodigiosin and Its Potential Applications in Agriculture. Agronomy 2022, 12, 3099. [Google Scholar] [CrossRef]
- Liu, X.; Shen, J.; Wang, Y.; Wu, J.; Lu, Z. Biological Dye Containing Prodigiosins, Preparation Method Thereof and Application Thereof. CN102199366A, 28 September 2011. [Google Scholar]
- Alihosseini, F.; Ju, K.S.; Lango, J.; Hammock, B.D.; Sun, G. Antibacterial Colorants: Characterization of Prodiginines and Their Applications on Textile Materials. Biotechnol. Prog. 2008, 24, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Kramar, A.; Ilic-Tomic, T.; Petkovic, M.; Radulović, N.; Kostic, M.; Jocic, D.; Nikodinovic-Runic, J. Crude Bacterial Extracts of Two New Streptomyces sp. Isolates as Bio-Colorants for Textile Dyeing. World J. Microbiol. Biotechnol. 2014, 30, 2231–2240. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Sun, S.; Zhu, C.; Xu, W.; Park, Y.; Zhou, H. Mutant Breeding of Serratia marcescens Strain for Enhancing Prodigiosin Production and Application to Textiles. Prep. Biochem. Biotechnol. 2013, 43, 271–284. [Google Scholar] [CrossRef]
- Vaidyanathan, J.; Bhathena-Langdana, Z.; Adivarekar, R.V.; Nerurkar, M. Production, Partial Characterization, and Use of a Red Biochrome Produced by Serratia sakuensis subsp. Nov Strain KRED for Dyeing Natural Fibers. Appl. Biochem. Biotechnol. 2012, 166, 321–335. [Google Scholar] [CrossRef]
- Namazkar, S.; Garg, R.; Ahmad, W.Z.; Nordin, N. Production and Characterization of Crude and Encapsulated Prodigiosin Pigment. Int. J. Chem. Sci. Appl. 2013, 4, 116–129. [Google Scholar]
- Burkinshaw, S.M.; Kumar, N. The Mordant Dyeing of Wool Using Tannic Acid and FeSO4, Part 1: Initial Findings. Dyes Pigm. 2009, 80, 53–60. [Google Scholar] [CrossRef]
- Mongkholrattanasit, R.; Kryštůfek, J.; Wiener, J.; Viková, M. UV Protection Properties of Silk Fabric Dyed with Eucalyptus Leaf Extract. J. Text. Inst. 2011, 102, 272–279. [Google Scholar] [CrossRef]
- Uddin, M.G. Effects of Different Mordants on Silk Fabric Dyed with Onion Outer Skin Extracts. J. Text. 2014, 2014, 405626. [Google Scholar] [CrossRef]
- Cai, C.; Li, F.; Liu, L.; Tan, Z. Deep Eutectic Solvents Used as the Green Media for the Efficient Extraction of Caffeine from Chinese Dark Tea. Sep. Purif. Technol. 2019, 227, 115723. [Google Scholar] [CrossRef]
- Torres-Vega, J.; Gómez-Alonso, S.; Pastene-Navarrete, E.; Pérez-Navarro, J. Green Extraction of Alkaloids and Polyphenols from Peumus boldus Leaves with Natural Deep Eutectic Solvents and Profiling by HPLC-PDA-IT-MS/MS and HPLC-QTOF-MS/MS. Plants 2020, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Shawky, E.; Takla, S.S.; Hammoda, H.M.; Darwish, F.A. Evaluation of the Influence of Green Extraction Solvents on the Cytotoxic Activities of Crinum (Amaryllidaeae) Alkaloid Extracts Using in-Vitro-in-Silico Approach. J. Ethnopharmacol. 2018, 227, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, B.A.; Remmele, R.L. Protect from Light: Photodegradation and Protein Biologics. J. Pharm. Sci. 2007, 96, 1468–1479. [Google Scholar] [CrossRef]
- Koch, R.; Fränz, J. Der Einfluss Des Lichtes Und Ionisierender Strahlung Auf Die Autoxydation von Cystein Und Die Reaktion Zwischen Cystein Und 3, 4-Benzpyren. Int. J. Radiat. Biol. 1960, 2, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Gong, J.; Fu, R.; Li, Z.; Yu, Z.; Lou, J.; Wang, F.; Zhang, J. Dyeing and Functional Properties of Polyester Fabric Dyed with Prodigiosins Nanomicelles Produced by Microbial Fermentation. J. Clean. Prod. 2017, 148, 375–385. [Google Scholar] [CrossRef]
- Gouveia, I.C. Nanobiotechnology: A New Strategy to Develop Non-Toxic Antimicrobial Textiles for Healthcare Applications. J. Biotechnol. 2010, 150, 349. [Google Scholar] [CrossRef]
- Mouro, C.; Martins, R.; Gomes, A.P.; Gouveia, I.C. Upcycling Wool Waste into Keratin Gel-Based Nanofibers Using Deep Eutectic Solvents. Gels 2023, 9, 661. [Google Scholar] [CrossRef] [PubMed]
- Akbar, N.; Khan, N.A.; Ibrahim, T.; Khamis, M.; Khan, A.S.; Alharbi, A.M.; Alfahemi, H.; Siddiqui, R. Antimicrobial Activity of Novel Deep Eutectic Solvents. Sci. Pharm. 2023, 91, 9. [Google Scholar] [CrossRef]
- Giteru, S.G.; Ramsey, D.H.; Hou, Y.; Cong, L.; Mohan, A.; Bekhit, A.E.D.A. Wool Keratin as a Novel Alternative Protein: A Comprehensive Review of Extraction, Purification, Nutrition, Safety, and Food Applications. Compr. Rev. Food Sci. Food Saf. 2023, 22, 643–687. [Google Scholar] [CrossRef] [PubMed]







| (a) | Temperature (°C) | (b) | Salts | |||||
| 40 | 60 | |||||||
| Acetate | K/S | 0.49 | 0.47 | Acetate | K/S | 0.59 | ||
| dE | 0.10 | 0.07 | dE | 0.19 | ||||
| Cotton | K/S | 0.78 | 0.70 | Cotton | K/S | 0.25 | ||
| dE | 0.10 | 0.08 | dE | 0.16 | ||||
| Nylon | K/S | 1.17 | 1.20 | Nylon | K/S | 1.25 | ||
| dE | 0.16 | 0.04 | dE | 0.14 | ||||
| Polyester | K/S | 0.68 | 0.72 | Polyester | K/S | 0.39 | ||
| dE | 0.09 | 0.05 | dE | 0.07 | ||||
| Acrylic | K/S | 0.51 | 0.30 | Acrylic | K/S | 0.14 | ||
| dE | 0.06 | 0.03 | dE | 0.10 | ||||
| Wool | K/S | 1.56 | 1.38 | Wool | K/S | 1.60 | ||
| dE | 0.12 | 0.05 | dE | 0.05 | ||||
| Mordants | |||||||
|---|---|---|---|---|---|---|---|
| Ferrous Sulphate (FeSO4) | L-Cysteine (L-Cys) | ||||||
| 1.0% | 3.0% | 5.0% | 1.0% | 3.0% | 5.0% | ||
| Acetate | K/S | 0.75 | 1.65 | 6.43 | 0.47 | 0.63 | 0.70 |
| dE | 0.15 | 0.08 | 0.17 | 0.13 | 0.08 | 0.10 | |
| Cotton | K/S | 0.55 | 0.90 | 1.23 | 0.59 | 0.86 | 0.94 |
| dE | 0.14 | 0.11 | 0.43 | 0.04 | 0.04 | 0.09 | |
| Nylon | K/S | 1.39 | 2.02 | 6.81 | 1.12 | 1.72 | 1.61 |
| dE | 0.09 | 0.09 | 0.16 | 0.06 | 0.02 | 0.07 | |
| Polyester | K/S | 0.48 | 0.60 | 0.77 | 0.68 | 0.76 | 0.91 |
| dE | 0.10 | 0.15 | 0.30 | 0.07 | 0.04 | 0.19 | |
| Acrylic | K/S | 0.27 | 0.66 | 0.92 | 0.19 | 0.21 | 0.35 |
| dE | 0.09 | 0.06 | 0.55 | 0.05 | 0.02 | 0.12 | |
| Wool | K/S | 1.47 | 2.44 | 2.06 | 1.69 | 2.08 | 2.48 |
| dE | 0.10 | 0.11 | 0.96 | 0.06 | 0.04 | 0.11 | |
| pH | |||||||
|---|---|---|---|---|---|---|---|
| 4.0 | 8.3 | ||||||
| Control | 3.0% owf FeSO4 | 3.0% owf L-Cys | Control | 3.0% owf FeSO4 | 3.0% owf L-Cys | ||
| Acetate | K/S | 1.71 | 3.81 | 1.53 | 0.51 | 1.01 | 0.58 |
| dE | 1.21 | 0.19 | 1.16 | 0.11 | 0.12 | 0.11 | |
| Cotton | K/S | 0.86 | 1.46 | 0.83 | 0.90 | 1.21 | 0.97 |
| dE | 0.83 | 0.62 | 0.80 | 0.17 | 0.23 | 0.13 | |
| Nylon | K/S | 1.73 | 3.30 | 1.59 | 1.87 | 3.67 | 2.30 |
| dE | 1.01 | 0.13 | 1.00 | 0.09 | 0.11 | 0.05 | |
| Polyester | K/S | 1.10 | 1.11 | 0.97 | 0.95 | 1.28 | 0.97 |
| dE | 0.41 | 0.22 | 0.33 | 0.12 | 0.26 | 0.11 | |
| Acrylic | K/S | 0.57 | 1.04 | 0.61 | 0.24 | 0.42 | 0.26 |
| dE | 0.17 | 0.23 | 0.15 | 0.17 | 0.20 | 0.11 | |
| Wool | K/S | 2.33 | 3.22 | 2.04 | 2.38 | 3.11 | 2.49 |
| dE | 0.70 | 0.35 | 0.84 | 0.25 | 0.33 | 0.27 | |
| Gel-Based Deep Eutectic Solvent (DES) | ||
|---|---|---|
| ChCl/LA (1:2) | ||
| Acetate | K/S | 2.76 |
| dE | 0.02 | |
| Cotton | K/S | 1.60 |
| dE | 0.02 | |
| Nylon | K/S | 3.33 |
| dE | 0.01 | |
| Polyester | K/S | 1.22 |
| dE | 0.01 | |
| Acrylic | K/S | 1.55 |
| dE | 0.01 | |
| Wool | K/S | 1.60 |
| dE | 0.01 | |
| ∆L* | ∆a* | ∆b* | ∆E* | Fastness Index | |
|---|---|---|---|---|---|
| ∆Color | |||||
| Prodigionsin_3.0% owf L-Cys_Nylon_pH = 8.3 | 1.66 | −0.77 | 2.64 | 3.21 | 3 |
| Prodigiosin_3.0% owf L-Cys_Wool_pH = 8.3 | 3.31 | −6.56 | 2.86 | 7.88 | 2 |
| Prodigiosin_gel-based ChCl/LA (1:2) DES_Nylon | 11.12 | −1.15 | 1.09 | 10.77 | 1–2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouro, C.; Gomes, A.P.; Costa, R.V.; Moghtader, F.; Gouveia, I.C. The Sustainable Bioactive Dyeing of Textiles: A Novel Strategy Using Bacterial Pigments, Natural Antibacterial Ingredients, and Deep Eutectic Solvents. Gels 2023, 9, 800. https://doi.org/10.3390/gels9100800
Mouro C, Gomes AP, Costa RV, Moghtader F, Gouveia IC. The Sustainable Bioactive Dyeing of Textiles: A Novel Strategy Using Bacterial Pigments, Natural Antibacterial Ingredients, and Deep Eutectic Solvents. Gels. 2023; 9(10):800. https://doi.org/10.3390/gels9100800
Chicago/Turabian StyleMouro, Cláudia, Ana P. Gomes, Rita V. Costa, Farzaneh Moghtader, and Isabel C. Gouveia. 2023. "The Sustainable Bioactive Dyeing of Textiles: A Novel Strategy Using Bacterial Pigments, Natural Antibacterial Ingredients, and Deep Eutectic Solvents" Gels 9, no. 10: 800. https://doi.org/10.3390/gels9100800