Enhancing Mechanical Performance of a Polymer Material by Incorporating Pillar[5]arene-Based Host–Guest Interactions
Abstract
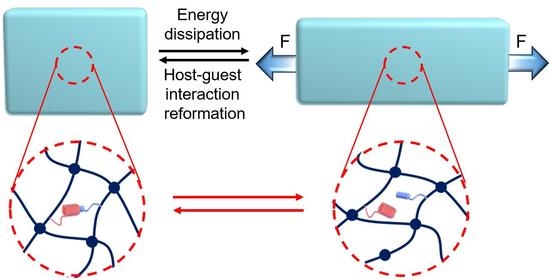
:1. Introduction
2. Results and Discussion
2.1. The Tensile Tests of the Gels
2.2. The Rheological Experiment of the Gels
2.3. The Swelling Experiment of the Gels
3. Conclusions
4. Materials and Methods
4.1. Materials and Instruments
4.2. Synthesis and Characterization of Compounds 3 and 4 and Guest 2
4.2.1. Synthesis and Characterization of Compound 3
4.2.2. Synthesis and Characterization of Compound 4
4.3. Synthesis and Characterization of Guest 2
4.4. Synthesis of G-HG, G-H, and G-G Gels
4.4.1. Synthesis of Gel G-HG
4.4.2. Synthesis of Gel G-H
4.4.3. Synthesis of Gel G-G
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Rodrigues, J.; Tomas, H. Injectable and biodegradable hydrogels: Gelation, biodegradation and biomedical applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef]
- Saracino, G.A.; Cigognini, D.; Silva, D.; Caprini, A.; Gelain, F. Nanomaterials design and tests for neural tissue engineering. Chem. Soc. Rev. 2013, 42, 225–262. [Google Scholar] [CrossRef] [PubMed]
- Imato, K.; Ohishi, T.; Nishihara, M.; Takahara, A.; Otsuka, H. Network Reorganization of Dynamic Covalent Polymer Gels with Exchangeable Diarylbibenzofuranone at Ambient Temperature. J. Am. Chem. Soc. 2014, 136, 11839–11845. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Jie, K.; Zimmerman, S.C.; Huang, F. A double supramolecular crosslinked polymer gel exhibiting macroscale expansion and contraction behavior and multistimuli responsiveness. Polym. Chem. 2015, 6, 1912–1917. [Google Scholar] [CrossRef]
- Li, G.; Wu, J.; Wang, B.; Yan, S.; Zhang, K.; Ding, J.; Yin, J. Self-Healing Supramolecular Self-Assembled Hydrogels Based on Poly(l-glutamic acid). Biomacromolecules 2015, 16, 3508–3518. [Google Scholar] [CrossRef]
- Tran-Thi, T.-H.; Dagnelie, R.; Crunaire, S.; Nicole, L. Optical chemical sensors based on hybrid organic–inorganic sol–gel nanoreactors. Chem. Soc. Rev. 2011, 40, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.D.; Steed, J.W. Gels with sense: Supramolecular materials that respond to heat, light and sound. Chem. Soc. Rev. 2016, 45, 6546–6596. [Google Scholar] [CrossRef] [Green Version]
- Raemdonck, K.; Braeckmans, K.; Demeester, J.; De Smedt, S.C. Merging the best of both worlds: Hybrid lipid-enveloped matrix nanocomposites in drug delivery. Chem. Soc. Rev. 2014, 43, 444–472. [Google Scholar] [CrossRef] [PubMed]
- Mayr, J.; Saldías, C.; Díaz, D. Release of small bioactive molecules from physical gels. Chem. Soc. Rev. 2018, 47, 1484–1515. [Google Scholar] [CrossRef]
- Fantner, G.E.; Hassenkam, T.; Kindt, J.H.; Weaver, J.C.; Birkedal, H.; Pechenik, L.; Cutroni, J.A.; Cidade, G.A.; Stucky, G.D.; Morse, D.E. Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nat. Mater. 2005, 4, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X. Multi-scale multi-mechanism design of tough hydrogels: Building dissipation into stretchy networks. Soft Matter 2014, 10, 672–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, M.A.; Kurokawa, T.; Gong, J.P. Super tough double network hydrogels and their application as biomaterials. Polymer 2012, 53, 1805–1822. [Google Scholar] [CrossRef]
- Rodin, M.; Li, J.; Kuckling, D. Dually cross-linked single networks: Structures and applications. Chem. Soc. Rev. 2021, 50, 8147–8177. [Google Scholar] [CrossRef]
- Liu, J.; Tan, C.S.Y.; Yu, Z.; Lan, Y.; Abell, C.; Scherman, O.A. Biomimetic supramolecular polymer networks exhibiting both toughness and self-recovery. Adv. Mater. 2017, 29, 1604951. [Google Scholar] [CrossRef]
- Neal, J.A.; Mozhdehi, D.; Guan, Z. Enhancing mechanical performance of a covalent self-healing material by sacrificial noncovalent bonds. J. Am. Chem. Soc. 2015, 137, 4846–4850. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, M.; Tang, D.; Yan, X.; Zhang, Z.; Zhou, Z.; Song, B.; Wang, H.; Li, X.; Yin, S.; et al. Fluorescent Metallacage-Core Supramolecular Polymer Gel Formed by Orthogonal Metal Coordination and Host–Guest Interactions. J. Am. Chem. Soc. 2018, 140, 7674–7680. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, F.; Shen, X.; He, T.; Qiu, H.; Yin, S.; Stang, P.J. Self-Healing Metallacycle-Cored Supramolecular Polymers Based on a Metal–Salen Complex Constructed by Orthogonal Metal Coordination and Host–Guest Interaction with Amino Acid Sensing. ACS Macro Lett. 2021, 10, 873–879. [Google Scholar] [CrossRef]
- Peng, H.-Q.; Sun, C.-L.; Niu, L.-Y.; Chen, Y.-Z.; Wu, L.-Z.; Tung, C.-H.; Yang, Q.-Z. Supramolecular Polymeric Fluorescent Nanoparticles Based on Quadruple Hydrogen Bonds. Adv. Funct. Mater. 2016, 26, 5483–5489. [Google Scholar] [CrossRef]
- Peng, H.-Q.; Xu, J.-F.; Chen, Y.-Z.; Wu, L.-Z.; Tung, C.-H.; Yang, Q.-Z. Water-dispersible nanospheres of hydrogen-bonded supramolecular polymers and their application for mimicking light-harvesting systems. Chem. Commun. 2014, 50, 1334–1337. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Gu, J.; Wang, H.; Sessler, J.L.; Thordarson, P.; Lin, Y.-J.; Gong, H. AAAA–DDDD Quadruple H-Bond-Assisted Ionic Interactions: Robust Bis(guanidinium)/Dicarboxylate Heteroduplexes in Water. J. Am. Chem. Soc. 2019, 141, 20146–20154. [Google Scholar] [CrossRef]
- Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.-a.; Nakamoto, Y. para-Bridged Symmetrical Pillar[5]arenes: Their Lewis Acid Catalyzed Synthesis and Host–Guest Property. J. Am. Chem. Soc. 2008, 130, 5022–5023. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zheng, B.; Yao, Y.; Han, C.; Yuan, J.; Antonietti, M.; Huang, F. LCST-Type Phase Behavior Induced by Pillar[5]arene/Ionic Liquid Host–Guest Complexation. Adv. Mater. 2013, 25, 6864–6867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qiu, H.; He, T.; Li, Y.; Yin, S. Fluorescent Supramolecular Polymers Formed by Crown Ether-Based Host-Guest Interaction. Front. Chem. 2020, 8, 560. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, D.; Yan, X.; Chen, J.; Dong, S.; Zheng, B.; Huang, F. Self-Healing Supramolecular Gels Formed by Crown Ether Based Host–Guest Interactions. Angew. Chem. Int. Ed. 2012, 51, 7011–7015. [Google Scholar] [CrossRef]
- Lin, P.; Ma, S.; Wang, X.; Zhou, F. Molecularly Engineered Dual-Crosslinked Hydrogel with Ultrahigh Mechanical Strength, Toughness, and Good Self-Recovery. Adv. Mater. 2015, 27, 2054–2059. [Google Scholar] [CrossRef] [PubMed]
- Kean, Z.S.; Hawk, J.L.; Lin, S.; Zhao, X.; Sijbesma, R.P.; Craig, S.L. Increasing the Maximum Achievable Strain of a Covalent Polymer Gel Through the Addition of Mechanically Invisible Cross-Links. Adv. Mater. 2014, 26, 6013–6018. [Google Scholar] [CrossRef] [PubMed]
- Kakuta, T.; Takashima, Y.; Sano, T.; Nakamura, T.; Kobayashi, Y.; Yamaguchi, H.; Harada, A. Adhesion between semihard polymer materials containing cyclodextrin and adamantane based on host–guest interactions. Macromolecules 2015, 48, 732–738. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Yan, K.; Chen, J.; Xia, M.; Li, M.; Liu, K.; Wang, D.; Wu, C.; Xie, Y. Recent advances in novel aerogels through the hybrid aggregation of inorganic nanomaterials and polymeric fibers for thermalinsulation. Aggregate 2021, 2, e30. [Google Scholar] [CrossRef]
- Yao, L.; Ming, X.; Lin, C.; Duan, X.; Zhu, H.; Zhu, S.; Zhang, Q. Unusual switching of ionic conductivity in ionogels enabled by water-induced phase separation. Aggregate 2022, 3, e249. [Google Scholar] [CrossRef]
- Cao, D.; Kou, Y.; Liang, J.; Chen, Z.; Wang, L.; Meier, H. A Facile and Efficient Preparation of Pillararenes and a Pillarquinone. Angew. Chem. Int. Ed. 2009, 48, 9721–9723. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Wang, P.; Ji, X.; Khashab, N.M.; Sessler, J.L.; Huang, F. Functional Supramolecular Polymeric Networks: The Marriage of Covalent Polymers and Macrocycle-Based Host-Guest Interactions. Chem. Rev. 2020, 120, 6070–6123. [Google Scholar] [CrossRef]
- Ogoshi, T.; Sueto, R.; Yoshikoshi, K.; Yasuhara, K.; Yamagishi, T. Spherical Vesicles Formed by Co-Assembly of Cyclic Pentagonal Pillar[5]quinone with Cyclic Hexagonal Pillar[6]arene. J. Am. Chem. Soc. 2016, 138, 8064–8067. [Google Scholar] [CrossRef]
- Ogoshi, T.; Yamagishi, T.-A.; Nakamoto, Y. Pillar-Shaped Macrocyclic Hosts Pillar[n]arenes: New Key Players for Supramolecular Chemistry. Chem. Rev. 2016, 116, 7937–8002. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Yang, Y.; Chi, X.; Zhang, Z.; Huang, F. Pillararenes, a New Class of Macrocycles for Supramolecular Chemistry. Acc. Chem. Res. 2012, 45, 1294–1308. [Google Scholar] [CrossRef]
- Jie, K.; Zhou, Y.; Li, E.; Huang, F. Nonporous Adaptive Crystals of Pillararenes. Acc. Chem. Res. 2018, 51, 2064–2072. [Google Scholar] [CrossRef]
- Kakuta, T.; Yamagishi, T.; Ogoshi, T. Stimuli-Responsive Supramolecular Assemblies Constructed from Pillar[n]arenes. Acc. Chem. Res. 2018, 51, 1656–1666. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, X.-Y.; Li, Y.; Zou, X.; Xiong, S.; Lin, C.; Shen, Y.-Z.; Wang, L. Multistimuli-Responsive Supramolecular Vesicles Based on Water-Soluble Pillar[6]arene and SAINT Complexation for Controllable Drug Release. J. Am. Chem. Soc. 2014, 136, 10762–10769. [Google Scholar] [CrossRef] [PubMed]
- Li, C. Pillararene-based supramolecular polymers: From molecular recognition to polymeric aggregates. Chem. Commun. 2014, 50, 12420–12433. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ping, G.; Li, C. Efficient complexation between pillar[5]arenes and neutral guests: From host–guest chemistry to functional materials. Chem. Commun. 2016, 52, 9858–9872. [Google Scholar] [CrossRef] [PubMed]
- Ping, G.; Wang, Y.; Shen, L.; Wang, Y.; Hu, X.; Chen, J.; Hu, B.; Cui, L.; Meng, Q.; Li, C. Highly efficient complexation of sanguinarine alkaloid by carboxylatopillar[6]arene: PKa shift, increased solubility and enhanced antibacterial activity. Chem. Commun. 2017, 53, 7381–7384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.-F.; Li, Z.; Lin, Q.; Yang, Y.-W. Functional supramolecular gels based on pillar[n]arene macrocycles. Nanoscale 2020, 12, 2180–2200. [Google Scholar] [CrossRef] [PubMed]
- Sathiyajith, C.; Shaikh, R.R.; Han, Q.; Zhang, Y.; Meguellati, K.; Yang, Y.-W. Biological and related applications of pillar[n]arenes. Chem. Commun. 2017, 53, 677–696. [Google Scholar] [CrossRef]
- Li, H.; Chen, D.-X.; Sun, Y.-L.; Zheng, Y.B.; Tan, L.-L.; Weiss, P.S.; Yang, Y.-W. Viologen-Mediated Assembly of and Sensing with Carboxylatopillar[5]arene-Modified Gold Nanoparticles. J. Am. Chem. Soc. 2013, 135, 1570–1576. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Li, Z.; Yang, J.; Wang, C.; Wu, J.-R.; Wang, Y.; Zhang, D.; Yang, Y.-W. Supramolecular Assembly-Induced Emission Enhancement for Efficient Mercury(II) Detection and Removal. J. Am. Chem. Soc. 2019, 141, 4756–4763. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.-Y.; Yang, Y.-W. Pyridine-Conjugated Pillar[5]arene: From Molecular Crystals of Blue Luminescence to Red-Emissive Coordination Nanocrystals. J. Am. Chem. Soc. 2021, 143, 11976–11981. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Zhang, N.; Xia, W.; Wu, X.; Yao, C.; Liu, X.; Hu, X.-Y.; Lin, C.; Wang, L. Dramatically Promoted Swelling of a Hydrogel by Pillar[6]arene–Ferrocene Complexation with Multistimuli Responsiveness. J. Am. Chem. Soc. 2016, 138, 6643–6649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Z.; Zhao, Y. Pillararene-based self-assembled amphiphiles. Chem. Soc. Rev. 2018, 47, 5491–5528. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-Y.; Wu, X.; Wang, S.; Chen, D.; Xia, W.; Lin, C.; Pan, Y.; Wang, L. Pillar[5]arene-based supramolecular polypseudorotaxane polymer networks constructed by orthogonal self-assembly. Polym. Chem. 2013, 4, 4292–4297. [Google Scholar] [CrossRef]
- Zhou, Y.; Jie, K.; Zhao, R.; Huang, F. Cis–Trans Selectivity of Haloalkene Isomers in Nonporous Adaptive Pillararene Crystals. J. Am. Chem. Soc. 2019, 141, 11847–11851. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, H.; Huang, F. Alkyl Chain Length-Selective Vapor-Induced Fluorochromism of Pillar[5]arene-Based Nonporous Adaptive Crystals. J. Am. Chem. Soc. 2019, 141, 13290–13294. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, J.; Li, E.; Zhou, Y.; Li, Q.; Huang, F. Separation of Monochlorotoluene Isomers by Nonporous Adaptive Crystals of Perethylated Pillar[5]arene and Pillar[6]arene. J. Am. Chem. Soc. 2019, 141, 17102–17106. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, G.; Li, Q.; Wang, M.; Huang, F. Separation of Benzene and Cyclohexane by Nonporous Adaptive Crystals of a Hybrid[3]arene. J. Am. Chem. Soc. 2020, 142, 2228–2232. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Ni, M.; Yao, C.; Wang, X.; Chen, D.; Lin, C.; Hu, X.-Y.; Wang, L. Responsive Gel-like Supramolecular Network Based on Pillar[6]arene–Ferrocenium Recognition Motifs in Polymeric Matrix. Macromolecules 2015, 48, 4403–4409. [Google Scholar] [CrossRef]
- Chang, J.; Zhao, Q.; Kang, L.; Li, H.; Xie, M.; Liao, X. Multiresponsive Supramolecular Gel Based on Pillararene-Containing Polymers. Macromolecules 2016, 49, 2814–2820. [Google Scholar] [CrossRef]
- Boominathan, M.; Kiruthika, J.; Arunachalam, M. Construction of anion-responsive crosslinked polypseudorotaxane based on molecular recognition of pillar[5]arene. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1508–1515. [Google Scholar] [CrossRef]
- Shao, L.; Sun, J.; Hua, B.; Huang, F. An AIEE fluorescent supramolecular cross-linked polymer network based on pillar[5]arene host–guest recognition: Construction and application in explosive detection. Chem. Commun. 2018, 54, 4866–4869. [Google Scholar] [CrossRef] [PubMed]
- Kardelis, V.; Li, K.; Nierengarten, I.; Holler, M.; Nierengarten, J.-F.; Adronov, A. Supramolecular Organogels Prepared from Pillar[5]arene-Functionalized Conjugated Polymers. Macromolecules 2017, 50, 9144–9150. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Z.; Wang, R.; Wang, L.; He, X.; Jiang, H.; Tang, H.; Cao, D.; Tang, B.Z. A Conjugated Polymeric Supramolecular Network with Aggregation-Induced Emission Enhancement: An Efficient Light-Harvesting System with an Ultrahigh Antenna Effect. Angew. Chem. Int. Ed. 2020, 59, 9908–9913. [Google Scholar] [CrossRef]
- Wang, X.-H.; Song, N.; Hou, W.; Wang, C.-Y.; Wang, Y.; Tang, J.; Yang, Y.-W. Supramolecular Polymer Systems: Efficient Aggregation-Induced Emission Manipulated by Polymer Host Materials. Adv. Mater. 2019, 31, 1970261. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-F.; Chen, P. Pillar[5]arene-Based Resilient Supramolecular Gel with Dual-Stimuli Responses and Self-Healing Properties. ACS Appl. Polym. Mater. 2019, 1, 2224–2229. [Google Scholar] [CrossRef]
- Han, M.C.; He, H.W.; Kong, W.K.; Dong, K.; Wang, B.Y.; Yan, X.; Wang, L.M.; Ning, X. High-performance Electret and Antibacterial Polypropylene Meltblown Nonwoven Materials Doped with Boehmite and ZnO Nanoparticles for Air Filtration. Fiber. Polym. 2022, 23, 1–9. [Google Scholar] [CrossRef]
- Ning, F.; He, G.; Sheng, C.; He, H.; Wang, J.; Zhou, R.; Ning, X. Yarn on yarn abrasion performance of high modulus polyethylene fiber improved by graphene/polyurethane composites coating. J. Eng. Fiber. Fabr. 2021, 16, 1–10. [Google Scholar] [CrossRef]
- Wang, Z.J.; Qiang, H.F. Mechanical properties of thermal aged HTPB composite solid propellant under confining pressure. Def. Technol. 2022, 18, 618–625. [Google Scholar] [CrossRef]
- Liang, F.; Liu, W.; Zhang, S.; Zhang, B.; Han, X. Preparation and properties of anti-infrared transparent thermalinsulating film based on polymethyl methacrylate. Energy 2020, 194, 116848–116854. [Google Scholar] [CrossRef]
- Xia, B.; Zheng, B.; Han, C.; Dong, S.; Zhang, M.; Hu, B.; Yu, Y.; Huang, F. A novel pH-responsive supramolecular polymer constructed by pillar[5]arene-based host–guest interactions. Polym. Chem. 2013, 4, 2019–2024. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Zhang, H.; Hu, Z.; Zhang, Y.; Ji, X. Enhancing Mechanical Performance of a Polymer Material by Incorporating Pillar[5]arene-Based Host–Guest Interactions. Gels 2022, 8, 475. https://doi.org/10.3390/gels8080475
Huang C, Zhang H, Hu Z, Zhang Y, Ji X. Enhancing Mechanical Performance of a Polymer Material by Incorporating Pillar[5]arene-Based Host–Guest Interactions. Gels. 2022; 8(8):475. https://doi.org/10.3390/gels8080475
Chicago/Turabian StyleHuang, Chengdi, Hanwei Zhang, Ziqing Hu, Youping Zhang, and Xiaofan Ji. 2022. "Enhancing Mechanical Performance of a Polymer Material by Incorporating Pillar[5]arene-Based Host–Guest Interactions" Gels 8, no. 8: 475. https://doi.org/10.3390/gels8080475