Simulated Investigation in Wormhole Expansion Law of Gelling Acid Etching and Its Influencing Factors in Deep Carbonate Reservoirs
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Injection Rate on the Morphology of Vermicular Foramen
2.2. Influence of Gelling Acid Properties
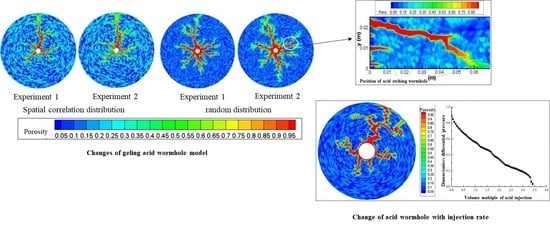
2.3. Effect of Pore Space Distribution on Wormhole Morphology
2.4. Effect of Perforation on Wormhole Shape
3. Conclusions
4. Materials and Methods
4.1. Continuity Equation
4.2. Equation of Motion
4.3. Gelling Acid Distribution Equation
4.4. Model Validation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hung, K.M.; Hill, A.D.; Sepehrnoorl, K. A mechanistic model of wormhole growth in carbonate matrix acidizing and acid fracturing. J. Pet. Technol. 1989, 41, 59–66. [Google Scholar] [CrossRef]
- Gdanski, R. A fundamentally new model of acid wormhole in carbonate. In Proceedings of the SPE European Formation Damage Conference, The Hague, The Netherlands, 31 May–1 June 1999. Paper SPE-54719-MS. [Google Scholar]
- Fredd, C.N.; Fogler, H.S. Influence of transport and reaction on wormhole formation in carbonate porous media. AIChE J. 1998, 44, 1933–1949. [Google Scholar] [CrossRef] [Green Version]
- Kang, Q.J.; Zhang, D.X.; Chen, S.Y.; He, X. Lattice Boltzmann simulation of chemical dissolution in porous media. Phys. Rev. E 2002, 65, 036318. [Google Scholar] [CrossRef] [Green Version]
- Golfier, F.; Zarcone, C.; Bazin, B.; Lenormand, R.; Lasseux, D.; Quintard, M. On the ability of a Darcy scale model to capture wormhole formation during the dissolution of a porous medium. J. Fluid Mech. 2002, 457, 213–254. [Google Scholar] [CrossRef]
- Ahmadi, M.; Panahi, F.; Bahri-Laleh, N.; Sabzi, M.; Pareras, G.; Falcone, B.N.; Poater, A. pH-responsive gelation in metallo-supramolecular polymers based on the protic pyridinedicarboxamide ligand. Chem. Mater. 2022, 34, 6155–6169. [Google Scholar] [CrossRef]
- Huang, T.; Hill, A.D.; Schechter, R. Reaction rate and fluid loss: The keys to wormhole initiation and propagation in carbonate acidizing. SPE J. 2000, 5, 287–292. [Google Scholar] [CrossRef]
- Panga, M.K.; Ziauddin, M.; Balakotaiah, V. A new model for predicting wormhole structure and formation in acid stimulation of carbonates. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 18–20 February 2004. Paper SPE 86517. [Google Scholar]
- Panga, M.K.; Ziauddin, M.; Balakotaiah, V. Two-scale continuum model for simulation of wormholes in carbonate acidization. AIChE J. 2005, 51, 3231–3248. [Google Scholar] [CrossRef]
- Cohen, C.E.; Ding, D.; Quintard, M.; Bazin, B. From pore scale to wellbore scale: Impact of geometry on wormhole growth in carbonate acidization. Chem. Eng. Sci. 2007, 63, 3088–3099. [Google Scholar] [CrossRef]
- Akanni, O.O. Mathematical Modeling of Wormhole Propagation during Matrix Acidizing of Carbonate Reservoir; Texas A&M University: College Station, TX, USA, 2015. [Google Scholar]
- Akanni, O.O.; Nasr-El-Din, H.A. The accuracy of carbonate matrix-acidizing models in predicting optimum injection and wormhole propagation rates. In Proceedings of the SPE Middle East Oil & Gas Show and Conference, Manama, Bahrain, 8–11 March 2015. Paper SPE 172575. [Google Scholar]
- Olatokunbo, O.A.; Nasr-El-Din, H.A. Modeling of wormhole propagation during matrix acidizing of carbonate reservoirs by organic acids and chelating agents. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dubai, United Arab Emirates, 26–28 September 2016. Paper SPE-181348-MS. [Google Scholar]
- Liu, M.; Zhang, S.; Mou, J.Y. Effect of normally distributed porosity sities on dissolution pattern in carbonate acidizing. Pet. Sci. Eng. 2012, 94, 28–39. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Zhang, S.; Mou, J.Y. Wormhole propagation behavior and its effect on acid leakoff under in situ conditions in acid fracturing. Transp. Porous Media 2014, 101, 99–114. [Google Scholar] [CrossRef]
- Mou, J.Y.; Liu, M.; Zhang, S. Diversion conditions for viscoelastic surfactant-based self-diversion acid in carbonate acidizing. SPE Prod. Oper. 2015, 30, 191–199. [Google Scholar] [CrossRef]
- Qiu, X.; Aidagulov, G.; Ghommem, M.; Edelman, E.; Brady, D.; Abbad, M. Towards a better understanding of wormhole propagation in carbonate rocks: Linear vs. radial acid injection. J. Pet. Sci. Eng. 2018, 171, 570–583. [Google Scholar] [CrossRef]
- Qi, D.; Zou, H.L.; Ding, Y.H.; Zhang, H.W. Wormhole calculation model of carbonate acidizing based on Hausdorff fractal dimension. Sci. Technol. Eng. 2019, 19, 105–110. [Google Scholar]
- Wang, L.; Mou, J.Y.; Mo, S.Q.; Zhao, B.; Liu, Z.Y.; Tian, X.X. Modeling matrix acidizing in naturally fractured carbonate reservoirs. J. Pet. Sci. Eng. 2020, 186, 106685. [Google Scholar] [CrossRef]
- Liu, N.Z.; Liu, M. Simulation and analysis of wormhole propagation by VES acid in carbonate acidizing. J. Pet. Sci. Eng. 2016, 138, 57–65. [Google Scholar] [CrossRef]
- Wei, W.; Yu, W.; Chen, Y.G.; Sepehrnoori, K. Geochemical modeling of wormhole propagation during carbonate acidizing with consideration of fractures. In Proceedings of the SPE Improved Oil Recovery Conference, Virtual, 31 August–September 2020. Paper SPE-200319-MS. [Google Scholar]
- Qi, N.; Chen, G.B.; Liang, C.; Guo, T.K.; Liu, G.L.; Zhang, K. Numerical simulation and analysis of the influence of fracture geometry on wormhole propagation in carbonate reservoirs. Chem. Eng. Sci. 2019, 198, 124–143. [Google Scholar] [CrossRef]
- Mustafa, A.; Alzaki, T.; Aljawad, M.S.; Solling, T.; Dvorkin, J. Impact of acid wormhole on the mechanical properties of chalk, limestone, and dolomite: Experimental and modeling studies. Energy Rep. 2022, 8, 605–616. [Google Scholar] [CrossRef]
- Ghommem, M.; Zhao, W.S.; Dyer, S.; Qiu, X.D.; Brady, D. Carbonate acidizing: Modeling, analysis, and characterization of wormhole formation and propagation. J. Pet. Sci. Eng. 2015, 131, 18–33. [Google Scholar] [CrossRef]
- Yoo, H.; Kim, Y.; Jang, H.; Lee, J. Propagation characteristics of optimum wormhole in carbonate matrix acidizing using micro X-ray CT imaging. J. Pet. Sci. Eng. 2021, 196, 108010. [Google Scholar] [CrossRef]
- Kiani, S.; Jafari, S.; Apourvari, S.N.; Mehrjoo, H. Simulation study of wormhole formation and propagation during matrix acidizing of carbonate reservoirs using a novel in-situ generated hydrochloric acid. Adv. Geo-Energy Res. 2021, 5, 64–74. [Google Scholar] [CrossRef]
- Elsafih, M.; Fahes, M. Quantifying the effect of multiphase flow on matrix acidizing in oil-Bearing carbonate formations. SPE Prod. Oper. 2021, 36, 795–806. [Google Scholar] [CrossRef]
- Nicolella, P.; Lauxen, D.; Ahmadi, M.; Seiffert, S. Reversible hydrogels with switchable diffusive permeability. Macromol. Chem. Phys. 2021, 222, 2100076. [Google Scholar] [CrossRef]
- Makeiff, D.A.; Cho, J.-Y.; Smith, B.; Carlini, R.; Godbert, N. Self-assembly of alkylamido isophthalic acids toward the design of a supergelator: Phase-selective gelation and dye adsorption. Gels 2022, 8, 285. [Google Scholar] [CrossRef]
- Wang, L.-L.; Wang, T.-F.; Wang, J.-X.; Tian, H.-T.; Chen, Y.; Song, W. Enhanced oil recovery mechanism and technical boundary of gel foam profile control system for heterogeneous reservoirs in Changqing. Gels 2022, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, J.; Dong, S.; Li, Y.; Slaný, M.; Chen, G. Use of Betaine-based gel and its potential application in enhanced oil recovery. Gels 2022, 8, 351. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Dai, C.; Sun, Y.; Zhao, G.; You, Q.; Liu, Y. Gelling behavior of PAM/phenolic crosslinked gel and its profile control in a low-temperature and high-salinity reservoir. Gels 2022, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Philippe, M.J.; Tardy, B.L.; Christanti, Y. An experimentally validated wormhole model for self-diverting and conventional acids in carbonate rocks under radial flow conditions. In Proceedings of the European Formation Damage Conference, Scheveningen, The Netherlands, 30 May–1 June 2007. Paper SPE-107854. [Google Scholar]


















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhou, W.; Li, S.; Wu, W. Simulated Investigation in Wormhole Expansion Law of Gelling Acid Etching and Its Influencing Factors in Deep Carbonate Reservoirs. Gels 2022, 8, 470. https://doi.org/10.3390/gels8080470
Wang M, Zhou W, Li S, Wu W. Simulated Investigation in Wormhole Expansion Law of Gelling Acid Etching and Its Influencing Factors in Deep Carbonate Reservoirs. Gels. 2022; 8(8):470. https://doi.org/10.3390/gels8080470
Chicago/Turabian StyleWang, Mingwei, Wen Zhou, Song Li, and Wen Wu. 2022. "Simulated Investigation in Wormhole Expansion Law of Gelling Acid Etching and Its Influencing Factors in Deep Carbonate Reservoirs" Gels 8, no. 8: 470. https://doi.org/10.3390/gels8080470