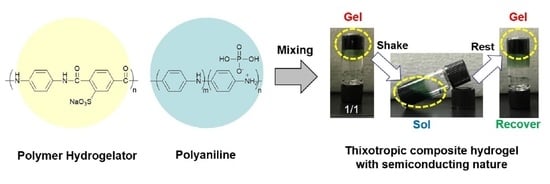
Polymeric Hydrogelator-Based Molecular Gels Containing Polyaniline/Phosphoric Acid Systems
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Reynolds, J.R.; Thompson, B.C.; Skotheim, T.A. Handbook of Conducting Polymers, 4th ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Chandrasekhar, P. Conducting Polymers, Fundamentals and Applications, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Rahman, M.A.; Kumar, P.; Park, D.-S.; Shim, Y.-B. Electrochemical Sensors Based on Organic Conjugated Polymers. Sensors 2008, 8, 118–141. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ahammad, A.J.S.; Jin, J.-H.; Ahn, S.J.; Lee, J.-J. Electrochemical DNA Hybridization Sensors Based on Conducting Polymers. Sensors 2015, 15, 3801–3829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.; Jawaid, M.; Khan, A.A.P.; Asiri, A.M. Electrically Conductive Polymers and Polymer Composites: From Synthesis to Biomedical Applications; Wiley-VCH: Weinheim, Germany, 2018. [Google Scholar]
- Le, T.H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, J.G.; Rincón, M.E.; Gutierrez-Granados, S.; Chahma, M.; Jaramillo-Quintero, O.A.; Frontana-Uribe, B.A. Conducting Polymers in the Fields of Energy, Environmental Remediation, and Chemical-Chiral Sensors. Chem. Rev. 2018, 118, 4731–4816. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting Polymers: A Comprehensive Review on Recent Advances in Synthesis, Properties and Applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Zare, E.N.; Makvandi, P.; Ashtari, B.; Rossi, F.; Motahari, A.; Perale, G. Progress in Conductive Polyaniline-Based Nanocomposites for Biomedical Applications: A Review. J. Med. Chem. 2020, 63, 1–22. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Rashid, S.A.; Shafie, S.; Sadrolhosseini, A.R.; Lim, H.N. Preparations, Properties, and Applications of Polyaniline and Polyaniline Thin Films—A Review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef]
- Pecher, J.; Mecking, S. Nanoparticles of Conjugated Polymers. Chem. Rev. 2010, 110, 6260–6279. [Google Scholar] [CrossRef]
- Jaymand, M. Recent progress in chemical modification of polyaniline. Prog. Polym. Sci. 2013, 38, 1287–1306. [Google Scholar] [CrossRef]
- Ma, Z.; Shi, W.; Yan, K.; Pan, P.; Yu, G. Doping engineering of conductive polymer hydrogels and their application in advanced sensor technologies. Chem. Sci. 2019, 10, 6232–6244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, M.H.; Vyas, C.; Grieve, B.; Bartolo, P. Recent Advances in Enzymatic and Non-Enzymatic Electrochemical Glucose Sensing. Sensors 2021, 21, 4672. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Inganäs, O. Conducting Polymer Hydrogels as 3D Electrodes: Applications for Supercapacitors. Adv. Mater. 1999, 11, 1214–1218. [Google Scholar] [CrossRef]
- Åsberg, P.; Inganäs, O. Hydrogels of a conducting conjugated polymer as 3-D enzyme electrode. Biosens. Bioelectron. 2003, 30, 199–207. [Google Scholar] [CrossRef]
- Pérez-Madrigal, M.M.; Estrany, F.; Armelin, E.; Díaz, D.D.; Alemán, C. Towards Sustainable Solid-State Supercapacitors: Electroactive Conducting Polymers Combined with Biohydrogels. J. Mater. Chem. A 2016, 4, 1792–1805. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Yu, G.; Zhai, D.; Lee, H.R.; Zhao, W.; Liu, N.; Wang, H.; Tee, B.C.K.; Shi, Y.; Cui, Y.; et al. Hierarchical Nanostructured Conducting Polymer Hydrogel with High Electrochemical Activity. Proc. Natl. Acad. Sci. USA 2012, 109, 9287–9292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, D.; Liu, B.; Shi, Y.; Pan, L.; Wang, Y.; Li, W.; Zhang, R.; Yu, G. Highly Sensitive Glucose Sensor Based on Pt Nanoparticle/Polyaniline Hydrogel Heterostructures. ACS Nano 2013, 7, 3540–3546. [Google Scholar] [CrossRef]
- Lu, Y.; He, W.; Cao, T.; Guo, H.; Zhang, Y.; Li, Q.; Shao, Z.; Cui, Y.; Zhang, X. Elastic, Conductive, Polymeric Hydrogels and Sponges. Sci. Rep. 2014, 4, 5792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, R.G.; Terech, P. Molecular Gels: Materials with Self-Assembled Fibrillar Networks; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Guenet, J.-M. Organogels Thermodynamics, Structure, Solvent Role, and Properties; Springer International Publishing AG: Cham, Switzerland, 2016. [Google Scholar]
- Weiss, R.G.; Blair, D.L.; Toro-Vazquez, J.F.; Perez-Martinez, J.D.; Li, J.; Zhang, Z.; Liu, X.; Rubio-Magnieto, J.; Escuder, B.; Rogers, M.A.; et al. Corradini. In Molecular Gels, Structure and Dynamics; The Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Dawn, A.; Shiraki, T.; Haraguchi, S.; Tamaru, S.; Shinkai, S. What Kind of “Soft Materials” Can We Design from Molecular Gels? Chem. Asian J. 2011, 6, 266–282. [Google Scholar] [CrossRef]
- Weiss, R.G. The Past, Present, and Future of Molecular Gels. What Is the Status of the Field, and Where Is It Going? J. Am. Chem. Soc. 2014, 136, 7519–7530. [Google Scholar] [CrossRef]
- Babu, S.S.; Praveen, V.K.; Ajayaghosh, A. Functional π-Gelators and Their Applications. Chem. Rev. 2014, 114, 1973–2129. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Weiss, R.G. Systematic Modifications of Alkane-Based Molecular Gelators and the Consequences to the Structures and Properties of Their Gels. New J. Chem. 2015, 39, 785–799. [Google Scholar] [CrossRef]
- Ohsedo, Y. Low-Molecular-Weight Organogelators as Functional Materials for Oil Spill Remediation. Polym. Adv. Technol. 2016, 27, 704–711. [Google Scholar] [CrossRef]
- Ohsedo, Y. Low-Molecular-Weight Gelators as Base Materials for Ointments. Gels 2016, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M. Development of C3-Symmetric Tris-Urea Low-Molecular-Weight Gelators. Chem. Rec. 2016, 16, 768–782. [Google Scholar] [CrossRef]
- Amabilino, D.B.; Smith, D.K.; Steed, J.W. Supramolecular Materials. Chem. Soc. Rev. 2017, 46, 2404–2420. [Google Scholar] [CrossRef]
- Miao, R.; Peng, J.; Fang, Y. Molecular Gels as Intermediates in the Synthesis of Porous Materials and Fluorescent Films: Concepts and Applications. Langmuir 2017, 33, 10419–10428. [Google Scholar] [CrossRef]
- Weiss, R.G. Controlling Variables in Molecular Gel Science: How Can We Improve the State of the Art? Gels 2018, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Mayr, J.; Saldías, C.; Díaz Díaz, D. Release of Small Bioactive Molecules from Physical Gels. Chem. Soc. Rev. 2018, 47, 1484–1515. [Google Scholar] [CrossRef]
- Chivers, P.R.A.; Smith, D.K. Shaping and Structuring Supramolecular Gels. Nat. Rev. Mater. 2019, 4, 463–478. [Google Scholar] [CrossRef] [Green Version]
- Panja, S.; Adams, D.J. Stimuli Responsive Dynamic Transformations in Supramolecular Gels. Chem. Soc. Rev. 2021, 50, 5165–5200. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Wojtecki, R.J.; Meador, M.A.; Rowan, S.J. Using the Dynamic Bond to Access Macroscopically Responsive Structurally Dynamic Polymers. Nat. Mater. 2011, 10, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Krieg, E.; Bastings, M.M.C.; Besenius, P.; Rybtchinski, B. Supramolecular Polymers in Aqueous Media. Chem. Rev. 2016, 116, 2414–2477. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.; Hughes, R. Rheology for Chemists: An Introduction, 2nd ed.; Royal Society of Chemistry: Cambridge, UK, 2008. [Google Scholar]
- Cornwell, D.J.; Smith, D.K. Expanding the scope of gels —combining polymers with low-molecular-weight gelators to yield modified self-assembling smart materials with high-tech applications. Mater. Horiz. 2015, 2, 279–293. [Google Scholar] [CrossRef] [Green Version]
- Guenet, J.M. Hybrid Physical Gels from Polymers and Self-Assembled Systems: A Novel Path for Making Functional Materials. Gels 2018, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Draper, E.R.; Adams, D.J. How Should Multicomponent Supramolecular Gels Be Characterised? Chem. Soc. Rev. 2018, 47, 3395–3405. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, D.; Srinivasan, S.; Rochas, C.; Ajayaghosh, A.; Guenet, J.M. Hybrid Thermoreversible Gels from Covalent Polymers and Organogels. Langmuir 2009, 25, 8593–8598. [Google Scholar] [CrossRef]
- Nyrkova, I.; Moulin, E.; Armao, I.J.J.; Maaloum, M.; Heinrich, B.; Rawiso, M.; Niess, F.; Cid, J.-J.; Jouault, N.; Buhler, E.; et al. Supramolecular Self-Assembly and Radical Kinetics in Conducting Self-Replicating Nanowires. ACS Nano 2014, 8, 10111–10124. [Google Scholar] [CrossRef]
- Zoukal, Z.; Elhasri, S.; Carvalho, A.; Schmutz, M.; Collin, D.; Vakayil, P.K.; Ajayaghosh, A.; Guenet, J.M. Hybrid Materials from Poly(Vinyl Chloride) and Organogels. ACS Appl. Polym. Mater. 2019, 1, 1203–1208. [Google Scholar] [CrossRef]
- Talebpour, P.; Heinrich, B.; Gavat, O.; Carvalho, A.; Moulin, E.; Giuseppone, N.; Guenet, J.M. Modulation of the Molecular Structure of Tri-Aryl Amine Fibrils in Hybrid Poly[Vinyl Chloride] Gel/Organogel Systems. Macromolecules 2021, 54, 8104–8111. [Google Scholar] [CrossRef]
- Ohsedo, Y.; Watanabe, H.; Oono, M.; Tanaka, A. Mixing Enhancement Effect of Low-Molecular-Weight Organogelators for Thixotropic Organogel Creation. Chem. Lett. 2013, 42, 363–365. [Google Scholar] [CrossRef]
- Ohsedo, Y.; Oono, M.; Tanaka, A.; Watanabe, H. Mixing Induced Thixotropy of a Two-Component System of Alkylurea Organogelators Having Different Alkyl Chains. New J. Chem. 2013, 37, 2250–2253. [Google Scholar] [CrossRef]
- Ohsedo, Y.; Oono, M.; Saruhashi, K.; Watanabe, H.; Miyamoto, N. A New Composite Thixotropic Hydrogel Composed of a Low-Molecular-Weight Hydrogelator and a Nanosheet. RSC Adv. 2014, 4, 44837–44840. [Google Scholar] [CrossRef]
- Ohsedo, Y.; Oono, M.; Saruhashi, K.; Watanabe, H. A New Water-Soluble Aromatic Polyamide Hydrogelator with Thixotropic Properties. RSC Adv. 2015, 5, 82772–82776. [Google Scholar] [CrossRef]
- Ohsedo, Y.; Oono, M.; Saruhashi, K.; Watanabe, H.; Miyamoto, N. New Composite Thixotropic Hydrogel Composed of a Polymer Hydrogelator and a Nanosheet. R. Soc. Open Sci. 2017, 4, 171117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohsedo, Y.; Saruhashi, K.; Watanabe, H.; Miyamoto, N. Synthesis of an Electronically Conductive Hydrogel from a Hydrogelator and a Conducting Polymer. New J. Chem. 2017, 41, 9602–9606. [Google Scholar] [CrossRef]
- Amaya, T.; Sugihara, R.; Hata, D.; Hirao, T. Self-Doped Polyaniline Derived from Poly(2-Methoxyaniline-5-Phosphonic Acid) and Didodecyldimethylammonium Salt. RSC Adv. 2016, 6, 22447–22452. [Google Scholar] [CrossRef] [Green Version]
- Amaya, T.; Kurata, I.; Inada, Y.; Hatai, T.; Hirao, T. Synthesis of Phosphonic Acid Ring-Substituted Polyanilines via Direct Phosphonation to Polymer Main Chains. RSC Adv. 2017, 7, 39306–39313. [Google Scholar] [CrossRef] [Green Version]
- Kavanagh, G.M.; Ross-Murphy, S.B. Rheological Characterisation of Polymer Gels. Prog. Polym. Sci. 1998, 23, 533–562. [Google Scholar] [CrossRef]
- Dawn, A.; Kumari, H. Low Molecular Weight Supramolecular Gels Under Shear: Rheology as the Tool for Elucidating Structure–Function Correlation. Chem.–A Eur. J. 2018, 24, 762–776. [Google Scholar] [CrossRef]
- Aston, R.; Sewell, K.; Klein, T.; Lawrie, G.; Grøndahl, L. Evaluation of the Impact of Freezing Preparation Techniques on the Characterisation of Alginate Hydrogels by Cryo-SEM. Eur. Polym. J. 2016, 82, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.S.; MacDiarmid, A.G. Optical Properties of Polyaniline. Polymer 1993, 34, 1833–1845. [Google Scholar] [CrossRef]
- Watanabe, A.; Mori, K.; Iwasaki, Y.; Nakamura, Y.; Niizuma, S. Electrochromism of Polyaniline Film Prepared by Electrochemical Polymerization. Macromolecules 1987, 20, 1793–1796. [Google Scholar] [CrossRef]
- Shirota, Y.; Noma, N.; Kanega, H.; Mikawa, H. Preparation of an Electrically Conducting Polymer by the Electrolytic Polymerization of N-Vinylcarbazole. J. Chem. Soc. Chem. Commun. 1984, 7, 470–471. [Google Scholar] [CrossRef]
- Kakuta, T.; Shirota, Y.; Mikawa, H. A Rechargeable Battery Using Electrochemically Doped Poly(N-Vinylcarbazole). J. Chem. Soc. Chem. Commun. 1985, 9, 553–554. [Google Scholar] [CrossRef]
- Rubinstein, I.; Sabatani, E.; Rishpon, J. Electrochemical Impedance Analysis of Polyaniline Films on Electrodes. J. Electrochem. Soc. 1987, 134, 3078–3083. [Google Scholar] [CrossRef]
- Viale, S.; Best, A.S.; Mendes, E.; Jager, W.F.; Picken, S.J. A Supramolecular Nematic Phase in Sulfonated Polyaramides. Chem. Commun. 2004, 12, 1596–1597. [Google Scholar] [CrossRef]
- Viale, S.; Li, N.; Schotman, A.H.M.; Best, A.S.; Picken, S.J. Synthesis and Formation of a Supramolecular Nematic Liquid Crystal in Poly(p-Phenylene−sulfoterephthalamide)−H2O. Macromolecules 2005, 38, 3647–3652. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohsedo, Y.; Sasaki, M. Polymeric Hydrogelator-Based Molecular Gels Containing Polyaniline/Phosphoric Acid Systems. Gels 2022, 8, 469. https://doi.org/10.3390/gels8080469
Ohsedo Y, Sasaki M. Polymeric Hydrogelator-Based Molecular Gels Containing Polyaniline/Phosphoric Acid Systems. Gels. 2022; 8(8):469. https://doi.org/10.3390/gels8080469
Chicago/Turabian StyleOhsedo, Yutaka, and Mayumi Sasaki. 2022. "Polymeric Hydrogelator-Based Molecular Gels Containing Polyaniline/Phosphoric Acid Systems" Gels 8, no. 8: 469. https://doi.org/10.3390/gels8080469