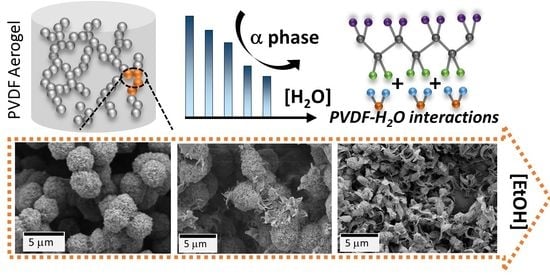
Polyvinylidene Fluoride Aerogels with Tailorable Crystalline Phase Composition
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gregorio, R.; Ueno, E.M. Effect of Crystalline Phase, Orientation and Temperature on the Dielectric Properties of Poly (Vinylidene Fluoride) (PVDF). J. Mater. Sci. 1999, 34, 4489–4500. [Google Scholar] [CrossRef]
- Miao, L.; Liu, G.; Wang, J. Ag-Nanoparticle-Bearing Poly(Vinylidene Fluoride) Nanofiber Mats as Janus Filters for Catalysis and Separation. ACS Appl. Mater. Interfaces 2019, 11, 7397–7404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yao, C.; Yu, Y.; Hong, Z.; Zhi, M.; Wang, X. Mesoporous Piezoelectric Polymer Composite Films with Tunable Mechanical Modulus for Harvesting Energy from Liquid Pressure Fluctuation. Adv. Funct. Mater. 2016, 26, 6760–6765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, J.; Chang, S. PVDF-Based Ferroelectric Polymers and Dielectric Elastomers for Sensor and Actuator Applications: A Review. Funct. Compos. Struct. 2019, 1, 012003. [Google Scholar] [CrossRef]
- Wan, C.; Bowen, C.R. Multiscale-Structuring of Polyvinylidene Fluoride for Energy Harvesting: The Impact of Molecular-, Micro- and Macro-Structure. J. Mater. Chem. A 2017, 5, 3091–3128. [Google Scholar] [CrossRef] [Green Version]
- Soulestin, T.; Ladmiral, V.; Dos Santos, F.D.; Améduri, B. Vinylidene Fluoride- and Trifluoroethylene-Containing Fluorinated Electroactive Copolymers. How Does Chemistry Impact Properties? Prog. Polym. Sci. 2017, 72, 16–60. [Google Scholar] [CrossRef]
- Salimi, A.; Yousefi, A.A. Conformational Changes and Phase Transformation Mechanisms in PVDF Solution-Cast Films. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 3487–3495. [Google Scholar] [CrossRef]
- He, X.; Yao, K. Crystallization Mechanism and Piezoelectric Properties of Solution-Derived Ferroelectric Poly(Vinylidene Fluoride) Thin Films. Appl. Phys. Lett. 2006, 89, 112909. [Google Scholar] [CrossRef]
- Bansil, R.; Lal, J.; Carvalho, B.L. Effects of Gelation on Spinodal Decomposition Kinetics in Gelatin. Polymer 1992, 33, 2961–2969. [Google Scholar] [CrossRef]
- Lovinger, A.J. Ferroelectric Polymers. Science 1983, 220, 1115–1121. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Zhao, Y.; Yan, C.; Wang, S.; Yang, H.; Wang, X.; Schultz, J.M. Preparation of Gamma-PVDF with Controlled Orientation and Insight into Phase Transformation. Polymer 2017, 123, 282–289. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, W.; Tan, B.; Zhu, C.; Ni, Y.; Fang, L.; Lu, C.; Xu, Z. Crystallinity and β Phase Fraction of PVDF in Biaxially Stretched PVDF/PMMA Films. Polymers 2021, 13, 998. [Google Scholar] [CrossRef]
- Sajkiewicz, P.; Wasiak, A.; Goclowski, Z. Phase Transitions during Stretching of Poly(Vinylidene Fluoride). Eur. Polym. J. 1999, 35, 423–429. [Google Scholar] [CrossRef]
- Tang, C.W.; Li, B.; Sun, L.; Lively, B.; Zhong, W.H. The Effects of Nanofillers, Stretching and Recrystallization on Microstructure, Phase Transformation and Dielectric Properties in PVDF Nanocomposites. Eur. Polym. J. 2012, 48, 1062–1072. [Google Scholar] [CrossRef]
- El Mohajir, B.E.; Heymans, N. Changes in Structural and Mechanical Behaviour of PVDF with Processing and Thermomechanical Treatments. 1. Change in Structure. Polymer 2001, 42, 5661–5667. [Google Scholar] [CrossRef]
- Qiao, S.; Zhang, H.; Kang, S.; Quan, J.; Hu, Z.; Yu, J.; Wang, Y.; Zhu, J. Hydrophobic, Pore-Tunable Polyimide/Polyvinylidene Fluoride Composite Aerogels for Effective Airborne Particle Filtration. Macromol. Mater. Eng. 2020, 305, 2000129. [Google Scholar] [CrossRef]
- Pickford, T.; Gu, X.; Heeley, E.L.; Wan, C. Effects of an Ionic Liquid and Processing Conditions on the β-Polymorph Crystal Formation in Poly(Vinylidene Fluoride). CrystEngComm 2019, 21, 5418–5428. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Zhang, J.; Chen, S.; Wang, X. Crystalline Phase Formation of Poly(Vinylidene Fluoride) from Tetrahydrofuran/N,N-Dimethylformamide Mixed Solutions. J. Macromol. Sci. Part B Phys. 2008, 47, 434–449. [Google Scholar] [CrossRef]
- Gheorghiu, F.; Stanculescu, R.; Curecheriu, L.; Brunengo, E.; Stagnaro, P.; Tiron, V.; Postolache, P.; Buscaglia, M.T.; Mitoseriu, L. PVDF–Ferrite Composites with Dual Magneto-Piezoelectric Response for Flexible Electronics Applications: Synthesis and Functional Properties. J. Mater. Sci. 2020, 55, 3926–3939. [Google Scholar] [CrossRef]
- Bodkhe, S.; Rajesh, P.S.M.; Kamle, S.; Verma, V. Beta-Phase Enhancement in Polyvinylidene Fluoride through Filler Addition: Comparing Cellulose with Carbon Nanotubes and Clay. J. Polym. Res. 2014, 21, 434. [Google Scholar] [CrossRef]
- Santos, J.P.F.; da Silva, A.B.; Arjmand, M.; Sundararaj, U.; Bretas, R.E.S. Nanofibers of Poly(Vinylidene Fluoride)/Copper Nanowire: Microstructural Analysis and Dielectric Behavior. Eur. Polym. J. 2018, 101, 46–55. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Liu, Z.; Yang, H.; Yuan, C.; Wang, Y. Facilitated Phase Transformation of PVDF in Its Composite with an Ionic Liquid. Polymer 2021, 220, 123564. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, J.; Zhang, J.; Chen, S.; Wang, X. Crystallization Behavior of PVDF/PMMA Blends Prepared by in Situ Polymerization from DMF and Ethanol. J. Mater. Sci. 2012, 47, 3720–3728. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, S.; Zhang, J.; Zhang, W.; Wang, X. Crystallization of PVDF in the PVDF/PMMA Blends Precipitated from Their Non-Solvents: Special Orientation Behavior, Morphology, and Thermal Properties. J. Cryst. Growth 2011, 328, 74–80. [Google Scholar] [CrossRef]
- Wei, D.; Zhou, S.; Li, M.; Xue, A.; Zhang, Y.; Zhao, Y.; Zhong, J.; Yang, D. PVDF/Palygorskite Composite Ultrafiltration Membranes: Effects of Nano-Clay Particles on Membrane Structure and Properties. Appl. Clay Sci. 2019, 181, 105171. [Google Scholar] [CrossRef]
- Zhou, Z.; Du, X.; Luo, J.; Yao, L.; Zhang, Z.; Yang, H.; Zhang, Q. Coupling of Interface Effects and Porous Microstructures in Translucent Piezoelectric Composites for Enhanced Energy Harvesting and Sensing. Nano Energy 2021, 84, 105895. [Google Scholar] [CrossRef]
- Cardea, S.; Gugliuzza, A.; Sessa, M.; Aceto, M.C.; Drioli, E.; Reverchon, E. Supercritical Gel Drying: A Powerful Tool for Tailoring Symmetric Porous PVDF-HFP Membranes. ACS Appl. Mater. Interfaces 2009, 1, 171–180. [Google Scholar] [CrossRef]
- Cardea, S.; Sessa, M.; Reverchon, E. Processing of Co-Crystalline and Nanoporous-Crystalline Polymers: Supercritical Co2 Processing of Drug Loaded Membranes Based on Nanoporous PVDF-HFP Aerogels. Soft Mater. 2011, 9, 264–279. [Google Scholar] [CrossRef]
- Cardea, S.; Sessa, M.; Reverchon, E. Supercritical CO2 Assisted Formation of Poly(Vinylidenefluoride) Aerogels Containing Amoxicillin, Used as Controlled Release Device. J. Supercrit. Fluids 2011, 59, 149–156. [Google Scholar] [CrossRef]
- Li, J.; Tenjimbayashi, M.; Zacharia, N.S.; Shiratori, S. One-Step Dipping Fabrication of Fe3O4/PVDF-HFP Composite 3D Porous Sponge for Magnetically Controllable Oil-Water Separation. ACS Sustain. Chem. Eng. 2018, 6, 10706–10713. [Google Scholar] [CrossRef]
- Chen, F.; Lu, Y.; Liu, X.; Song, J.; He, G.; Tiwari, M.K.; Carmalt, C.J.; Parkin, I.P. Table Salt as a Template to Prepare Reusable Porous PVDF–MWCNT Foam for Separation of Immiscible Oils/Organic Solvents and Corrosive Aqueous Solutions. Adv. Funct. Mater. 2017, 27, 1702926. [Google Scholar] [CrossRef]
- Chen, X.; Liang, Y.N.; Tang, X.Z.; Shen, W.; Hu, X. Additive-Free Poly (Vinylidene Fluoride) Aerogel for Oil/Water Separation and Rapid Oil Absorption. Chem. Eng. J. 2017, 308, 18–26. [Google Scholar] [CrossRef]
- Seraji, S.M.; Jin, X.; Yi, Z.; Feng, C.; Salim, N.V. Ultralight Porous Poly (Vinylidene Fluoride)-Graphene Nanocomposites with Compressive Sensing Properties. Nano Res. 2021, 14, 2620–2629. [Google Scholar] [CrossRef]
- Cheraghi Bidsorkhi, H.; D’Aloia, A.G.; Tamburrano, A.; De Bellis, G.; Delfini, A.; Ballirano, P.; Sarto, M.S. 3D Porous Graphene Based Aerogel for Electromagnetic Applications. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Zhang, Y.; Yu, Z.; Zheng, J.; Wang, Y.; Zhou, H. PANI/PVDF-TrFE Porous Aerogel Bulk Piezoelectric and Triboelectric Hybrid Nanogenerator Based on in-Situ Doping and Liquid Nitrogen Quenching. Nano Energy 2021, 80, 105519. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, Y.; Shen, X. Polyvinylidene Fluoride Aerogel with High Thermal Stability and Low Thermal Conductivity. Mater. Lett. 2020, 259, 126890. [Google Scholar] [CrossRef]
- Kim, S.J.; Raut, P.; Jana, S.C.; Chase, G. Electrostatically Active Polymer Hybrid Aerogels for Airborne Nanoparticle Filtration. ACS Appl. Mater. Interfaces 2017, 9, 6401–6410. [Google Scholar] [CrossRef]
- Ewa Piorkowska, G.C.R. Handbook of Polymer Crystallization; Piorkowska, E., Rutledge, G.C., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; ISBN 9781118541838. [Google Scholar]
- Cheng, L.P.; Young, T.H.; Fang, L.; Gau, J.J. Formation of Particulate Microporous Poly(Vinylidene Fluoride) Membranes by Isothermal Immersion Precipitation from the 1-Octanol/Dimethylformamide/Poly(Vinylidene Fluoride) System. Polymer 1999, 40, 2395–2403. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A Critical Analysis of the α, β and γ Phases in Poly(Vinylidene Fluoride) Using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef]
- Tanigami, T.; Suzuki, H.; Yamaura, K.; Matsuzawa, S. Gelation and Crystallization of Poly (4-Methyl-1-Pentene) in Cyclohexane Solution. Macromolecules 1985, 18, 2595–2600. [Google Scholar] [CrossRef]
- Coniglio, A.; Stanley, H.E.; Klein, W. Site-Bond Correlated-Percolation Problem: A Statistical Mechanical Model of Polymer Gelation. Phys. Rev. Lett. 1979, 42, 518–522. [Google Scholar] [CrossRef]
- Díaz de los Ríos, M.; Hernández Ramos, E. Determination of the Hansen Solubility Parameters and the Hansen Sphere Radius with the Aid of the Solver Add-in of Microsoft Excel. SN Appl. Sci. 2020, 2, 676. [Google Scholar] [CrossRef] [Green Version]
- Charles, H. Hansen Solubility Parameters: A User´s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Bottino, A.; Capannelli, G.; Munari, S.; Turturro, A. Solubility Parameters of Poly(Vinylidene Fluoride). J. Polym. Sci. Part B Polym. Phys. 1988, 26, 785–794. [Google Scholar] [CrossRef]
- Novo, L.P.; Curvelo, A.A.S. Hansen Solubility Parameters: A Tool for Solvent Selection for Organosolv Delignification. Ind. Eng. Chem. Res. 2019, 58, 14520–14527. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, J.; Chen, S.; Zhang, J.; Wang, X. Controlled Crystallization of Poly(Vinylidene Fluoride) Chains from Mixed Solvents Composed of Its Good Solvent and Nonsolvent. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 575–581. [Google Scholar] [CrossRef]
- Bower, D. An Introduction to Polymer Physics; Cambridge University Press: Cambrigde, UK, 2002. [Google Scholar]
- Wu, Y.; Hsu, S.L.; Honeker, C.; Bravet, D.J.; Williams, D.S. The Role of Surface Charge of Nucleation Agents on the Crystallization Behavior of Poly(Vinylidene Fluoride). J. Phys. Chem. B 2012, 116, 7379–7388. [Google Scholar] [CrossRef]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation and Characterization of Membranes Formed by Nonsolvent Induced Phase Separation: A Review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Li, R.; Chen, C.; Li, J.; Xu, L.; Xiao, G.; Yan, D. A Facile Approach to Superhydrophobic and Superoleophilic Graphene/Polymer Aerogels. J. Mater. Chem. A 2014, 2, 3057–3064. [Google Scholar] [CrossRef]
- Jungnickel, J.B. Polymeric Materials Handbook; CRC Press Inc: New York, NY, USA, 1996; Volume 9. [Google Scholar]
- Shepelin, N.A.; Glushenkov, A.M.; Lussini, V.C.; Fox, P.J.; Dicinoski, G.W.; Shapter, J.G.; Ellis, A.V. New Developments in Composites, Copolymer Technologies and Processing Techniques for Flexible Fluoropolymer Piezoelectric Generators for Efficient Energy Harvesting. Energy Environ. Sci. 2019, 12, 1143–1176. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Kumar, A.; Sinha, A.N.; Kour, P.; Pandey, R.; Kumar, P.; Kar, M. Study of Ferroelectric Properties on PVDF-PZT Nanocomposite. Ferroelectrics 2017, 516, 18–27. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, K.; Zhou, Y.; Qu, Z.; Wang, H.; Zhang, Y.; Zhou, H.; Yan, C. Facile Preparation of Highly Oriented Poly(Vinylidene Fluoride) Uniform Films and Their Ferro- and Piezoelectric Properties. RSC Adv. 2017, 7, 17038–17043. [Google Scholar] [CrossRef] [Green Version]
- Boryczka, S.; Jastrzebska, M.; Bębenek, E.; Kusz, J.; Zubko, M.; Kadela, M.; Michalik, E. X-Ray Diffraction and Infrared Spectroscopy of N,N- Dimethylformamide and Dimethyl Sulfoxide Solvatomorphs of Betulonic Acid. J. Pharm. Sci. 2012, 101, 4458–4471. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Tashiro, K.; Tadokoro, H. Molecular Vibrations of Three Crystal Forms of Poly(Vinylidene Fluoride). Macromolecules 1975, 8, 158–171. [Google Scholar] [CrossRef]
- Tashiro, K.; Kobayashi, M.; Tadokoro, H. Vibrational Spectra and Disorder-Order Transition of Poly(Vinylidene Fluoride) Form III. Macromolecules 1981, 14, 1757–1764. [Google Scholar] [CrossRef]
- Tashiro, K.; Kobayashi, M. Structural Phase Transition in Ferroelectric Fluorine Polymers: X-Ray Diffraction and Infrared/Raman Spectroscopic Study. Phase Transit. 1989, 18, 213–246. [Google Scholar] [CrossRef]
- Dhevi, D.M.; Prabu, A.A.; Kim, K.J. Infrared Spectroscopic Studies on Crystalline Phase Transition of PVDF and PVDF/Hyperbranched Polyester Blend Ultrathin Films. Vib. Spectrosc. 2018, 94, 74–82. [Google Scholar] [CrossRef]
- Liu, Y.; Aziguli, H.; Zhang, B.; Xu, W.; Lu, W.; Bernholc, J.; Wang, Q. Ferroelectric Polymers Exhibiting Behaviour Reminiscent of a Morphotropic Phase Boundary. Nature 2018, 562, 96–100. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Tromba, G.; Longo, R.; Abrami, A.; Arfelli, F.; Astolfo, A.; Bregant, P.; Brun, F.; Casarin, K.; Chenda, V.; Dreossi, D.; et al. The SYRMEP Beamline of Elettra: Clinical Mammography and Bio-Medical Applications. In AIP Conference Proceedings; American institute of Physics: College Park, MD, USA, 2010; pp. 18–23. [Google Scholar]
- Brun, F.; Massimi, L.; Fratini, M.; Dreossi, D.; Billé, F.; Accardo, A.; Pugliese, R.; Cedola, A. SYRMEP Tomo Project: A Graphical User Interface for Customizing CT Reconstruction Workflows. Adv. Struct. Chem. Imaging 2017, 3, 4. [Google Scholar] [CrossRef]
- Paganin, D.; Mayo, S.C.; Gureyev, T.E.; Miller, P.R.; Wilkins, S.W. Simultaneous Phase and Amplitude Extraction from a Single Defocused Image of a Homogeneous Object. J. Microsc. 2002, 206, 33–40. [Google Scholar] [CrossRef]
- D’Amico, F.; Saito, M.; Bencivenga, F.; Marsi, M.; Gessini, A.; Camisasca, G.; Principi, E.; Cucini, R.; Di Fonzo, S.; Battistoni, A.; et al. UV Resonant Raman Scattering Facility at Elettra. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2013, 703, 33–37. [Google Scholar] [CrossRef]
- Birarda, G.; Bedolla, D.; Piccirilli, F.; Stani, C.; Vondracek, H.; Vaccari, L. Chemical Analyses at Micro and Nano Scale at SISSI-Bio Beamline at Elettra-Sincrotrone Trieste. In Proceedings of the Biomedical Vibrational Spectroscopy 2022: Advances in Research and Industry, San Francisco, CA, USA, 30 March 2022; Huang, Z., Ed.; SPIE: Bellingham, WA, USA, 2022; p. 31. [Google Scholar]
- Gregorio, R., Jr.; Cestari, M. Effect of Crystallization Temperature on the Crystalline Phase Content and Morphology of Poly(Vinylidene Fluoride). J. Polym. Sci. Part B Polym. Phys. 1994, 32, 859–870. [Google Scholar] [CrossRef]









| Sample | SBET (m2 g−1) a | Pore Diameter (nm) b | Pore Volume (cm3 g−1) c | tgel min d | (g cm−3) e | (g cm−3) f | Porosity (%) |
|---|---|---|---|---|---|---|---|
| P12 | 145 | 23 | 0.80 | 30 | 0.123 | 1.47 | 92 |
| P9 | 168 | 23 | 0.99 | 45 | 0.093 | 1.39 | 93 |
| P7 | - | - | - | No gel | - | - | - |
| P5 | - | - | - | No gel | - | - | - |
| P3 | - | - | - | No gel | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Rodriguez, J.; E. Bedolla, D.; D’Amico, F.; Koopmann, A.-K.; Vaccari, L.; Saccomano, G.; Kohns, R.; Huesing, N. Polyvinylidene Fluoride Aerogels with Tailorable Crystalline Phase Composition. Gels 2022, 8, 727. https://doi.org/10.3390/gels8110727
Torres-Rodriguez J, E. Bedolla D, D’Amico F, Koopmann A-K, Vaccari L, Saccomano G, Kohns R, Huesing N. Polyvinylidene Fluoride Aerogels with Tailorable Crystalline Phase Composition. Gels. 2022; 8(11):727. https://doi.org/10.3390/gels8110727
Chicago/Turabian StyleTorres-Rodriguez, Jorge, Diana E. Bedolla, Francesco D’Amico, Ann-Kathrin Koopmann, Lisa Vaccari, Giulia Saccomano, Richard Kohns, and Nicola Huesing. 2022. "Polyvinylidene Fluoride Aerogels with Tailorable Crystalline Phase Composition" Gels 8, no. 11: 727. https://doi.org/10.3390/gels8110727