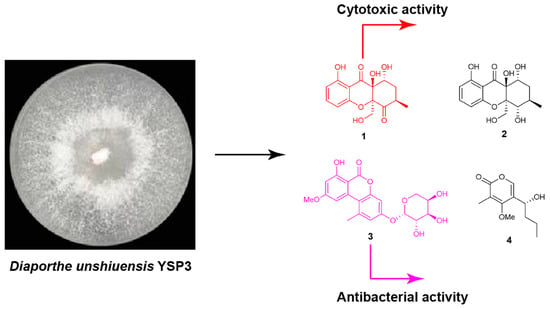
Chemical Investigation of Endophytic Diaporthe unshiuensis YSP3 Reveals New Antibacterial and Cytotoxic Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Fungal Materials
2.3. Fermentation, Extraction, and Purification
2.4. Formation of the (S)- and (R)-MTPA Esters of 4
2.5. Phomopthane A (1)
2.6. Phomopthane B (2)
2.7. Alternariol Methyl Ether-12-O-α-D-arabinoside (3)
2.8. Phomopyrone B (4)
2.9. X-ray Crystallographic Analysis
2.10. Crystal Data of 1
2.11. Crystal Data of 2
2.12. Antimicrobial Assays
2.13. Cytotoxic Assays
2.14. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dissanayake, A.J.; Chen, Y.-Y.; Liu, J.-K. Unravelling Diaporthe species associated with woody hosts from karst formations (Guizhou) in China. J. Fungi 2020, 6, 251. [Google Scholar] [CrossRef]
- Jia, L.-J.; Tang, H.-Y.; Wang, W.-Q.; Yuan, T.-L.; Wei, W.-Q.; Pang, B.; Gong, X.-M.; Wang, S.-F.; Li, Y.-J.; Zhang, D. A linear nonribosomal octapeptide from Fusarium graminearum facilitates cell-to-cell invasion of wheat. Nat. Commun. 2019, 10, 922. [Google Scholar] [CrossRef] [Green Version]
- Strobel, G.; Daisy, B.; Castillo, U.; Harper, J. Natural products from endophytic microorganisms. J. Nat. Prod. 2004, 67, 257–268. [Google Scholar] [CrossRef]
- Gomes, R.; Glienke, C.; Videira, S.; Lombard, L.; Groenewald, J.; Crous, P. Diaporthe: A genus of endophytic, saprobic and plant pathogenic fungi. Pers. -Mol. Phylogeny Evol. Fungi 2013, 31, 1–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udayanga, D.; Castlebury, L.A.; Rossman, A.Y.; Chukeatirote, E.; Hyde, K.D. Insights into the genus Diaporthe: Phylogenetic species delimitation in the D. eres species complex. Fungal Divers. 2014, 67, 203–229. [Google Scholar] [CrossRef] [Green Version]
- Tanney, J.B.; McMullin, D.R.; Green, B.D.; Miller, J.D.; Seifert, K.A. Production of antifungal and antiinsectan metabolites by the Picea endophyte Diaporthe maritima sp. nov. Fungal Biol. 2016, 120, 1448–1457. [Google Scholar] [CrossRef]
- Sebastianes, F.L.; Cabedo, N.; Aouad, N.E.; Valente, A.M.; Lacava, P.T.; Azevedo, J.L.; Pizzirani-Kleiner, A.A.; Cortes, D. 3-Hydroxypropionic acid as an antibacterial agent from endophytic fungi Diaporthe phaseolorum. Curr. Microbiol. 2012, 65, 622–632. [Google Scholar] [CrossRef]
- Sharma, V.; Singamaneni, V.; Sharma, N.; Kumar, A.; Arora, D.; Kushwaha, M.; Bhushan, S.; Jaglan, S.; Gupta, P. Valproic acid induces three novel cytotoxic secondary metabolites in Diaporthe sp., an endophytic fungus from Datura inoxia Mill. Bioorg. Med. Chem. Lett. 2018, 28, 2217–2221. [Google Scholar] [CrossRef]
- Cui, H.; Liu, Y.; Li, J.; Huang, X.; Yan, T.; Cao, W.; Liu, H.; Long, Y.; She, Z. Diaporindenes A–D: Four unusual 2, 3-dihydro-1 H-indene analogues with anti-inflammatory activities from the mangrove endophytic fungus Diaporthe sp. SYSU-HQ3. J. Org. Chem. 2018, 83, 11804–11813. [Google Scholar] [CrossRef]
- Niaz, S.I.; Khan, D.; Naz, R.; Safdar, K.; Abidin, S.Z.U.; Khan, I.U.; Gul, R.; Khan, W.U.; Khan, M.A.U.; Lan, L. Antimicrobial and antioxidant chlorinated azaphilones from mangrove Diaporthe perseae sp. isolated from the stem of Chinese mangrove Pongamia pinnata. J. Asian Nat. Prod. Res. 2021, 23, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Reveglia, P.; Pacetti, A.; Masi, M.; Cimmino, A.; Carella, G.; Marchi, G.; Mugnai, L.; Evidente, A. Phytotoxic metabolites produced by Diaporthe eres involved in cane blight of grapevine in Italy. Nat. Prod. Res. 2021, 35, 2872–2880. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yang, J.; Chen, F.; Lin, X.; Chen, C.; Zhou, X.; Liu, S.; Liu, Y. Structurally diverse polyketides from the mangrove-derived fungus Diaporthe sp. SCSIO 41011 with their anti-influenza A virus activities. Front. Chem. 2018, 6, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, T.-C.; Lu, Y.-H.; Wang, J.-F.; Song, Z.-Q.; Hou, Y.-G.; Liu, S.-S.; Liu, C.-S.; Wu, S.-H. Bioactive secondary metabolites of the genus Diaporthe and anamorph Phomopsis from terrestrial and marine habitats and endophytes: 2010–2019. Microorganisms 2021, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Pu, H.; Liu, J.; Wang, Y.; Peng, Y.; Zheng, W.; Tang, Y.; Hui, B.; Nie, C.; Huang, X.; Duan, Y. Bioactive α-pyrone derivatives from the endophytic fungus Diaporthe sp. CB10100 as inducible nitric oxide synthase inhibitors. Front. Chem. 2021, 9, 679592. [Google Scholar] [CrossRef]
- Zhao, X.; Li, K.; Zheng, S.; Yang, J.; Chen, C.; Zheng, X.; Wang, Y.; Ye, W. Diaporthe Diversity and Pathogenicity Revealed from a Broad Survey of Soybean Stem Blight in China. Plant Dis. 2022, 106, 2892–2903. [Google Scholar] [CrossRef] [PubMed]
- Flack, H.; Bernardinelli, G. The use of X-ray crystallography to determine absolute configuration. Chirality: Pharmacol. Biol. Chem. Conseq. Mol. Asymmetry 2008, 20, 681–690. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Sun, W.; Meng, J.; Wang, A.; Wang, X.; Tian, J.; Fu, X.; Dai, J.; Liu, Y.; Lai, D. Bioactive bis-naphtho-γ-pyrones from rice false smut pathogen Ustilaginoidea virens. J. Agric. Food Chem. 2015, 63, 3501–3508. [Google Scholar] [CrossRef]
- Yan, W.; Li, S.-J.; Guo, Z.-K.; Zhang, W.-J.; Wei, W.; Tan, R.-X.; Jiao, R.-H. New p-terphenyls from the endophytic fungus Aspergillus sp. YXf3. Bioorganic Med. Chem. Lett. 2017, 27, 51–54. [Google Scholar] [CrossRef]
- Khan, B.; Zhao, S.; Wang, Z.; Ye, Y.; Ahmed Rajput, N.; Yan, W. Eremophilane sesquiterpenes and benzene derivatives from the endophyte Microdiplodia sp. WGHS5. Chem. Biodivers. 2021, 18, e2000949. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, W.; Rui, X.; Chen, X.; Jiang, M.; Dong, M. Structural characterization and bioactivity of released exopolysaccharides from Lactobacillus plantarum 70810. Int. J. Biol. Macromol. 2014, 67, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, T.; Bao, C.; Liu, Z.; Fan, J.; Yang, R.; Qin, S. Design and synthesis of new norfloxacin-1, 3, 4-oxadiazole hybrids as antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Pharm. Sci. 2019, 136, 104966. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Tao, H.; Lin, X.; Wang, J.; Liao, S.; Dong, J.; Zhou, X.; Liu, Y. Prenylated indole alkaloids and chromone derivatives from the fungus Penicillium sp. SCSIO041218. Tetrahedron 2018, 74, 77–82. [Google Scholar] [CrossRef]
- Geng, C.-A.; Chen, X.-L.; Zhou, N.-J.; Chen, H.; Ma, Y.-B.; Huang, X.-Y.; Zhang, X.-M.; Chen, J.-J. LC-MS guided isolation of (±)-sweriledugenin A, a pair of enantiomeric lactones, from Swertia leducii. Org. Lett. 2014, 16, 370–373. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, N.; Wu, H.; Mei, Y.; Yang, J.; Tan, R. Cytotoxic and antibacterial polyketide-indole hybrids synthesized from indole-3-carbinol by Daldinia eschscholzii. Acta Pharm. Sin. B 2019, 9, 369–380. [Google Scholar] [CrossRef]
- Li, X.-B.; Chen, G.-Y.; Liu, R.-J.; Zheng, C.-J.; Song, X.-M.; Han, C.-R. A new biphenyl derivative from the mangrove endophytic fungus Phomopsis longicolla HL-2232. Nat. Prod. Res. 2017, 31, 2264–2267. [Google Scholar] [CrossRef]
- Liu, C.-C.; Zhang, Z.-Z.; Feng, Y.-Y.; Gu, Q.-Q.; Li, D.-H.; Zhu, T.-J. Secondary metabolites from Antarctic marine-derived fungus Penicillium crustosum HDN153086. Nat. Prod. Res. 2019, 33, 414–419. [Google Scholar] [CrossRef]
- Mondol, M.A.M.; Surovy, M.Z.; Islam, M.T.; Schüffler, A.; Laatsch, H. Macrocyclic trichothecenes from Myrothecium roridum strain M10 with motility inhibitory and zoosporicidal activities against Phytophthora nicotianae. J. Agric. Food Chem. 2015, 63, 8777–8786. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Chen, L.; Dong, Z.; Di, X.; Qiu, F. A new xanthone from Penicillium oxalicum. Chem. Nat. Compd. 2010, 46, 216–218. [Google Scholar] [CrossRef]
- Hanumaiah, T.; Rao, B.K.; Rao, C.; Rao, G.; Rao, J.; Rao, K.; Marshall, D.S.; Thomson, R.H. Naphthalenes and naphthoquinones from Ventilago species. Phytochemistry 1985, 24, 1811–1815. [Google Scholar] [CrossRef]
- Qiao, X.; Yin, J.; Yang, Y.; Zhang, J.; Shao, B.; Li, H.; Chen, H. Determination of Alternaria mycotoxins in fresh sweet cherries and cherry-based products: Method validation and occurrence. J. Agric. Food Chem. 2018, 66, 11846–11853. [Google Scholar] [CrossRef] [PubMed]
- Krick, A.; Kehraus, S.; Gerhäuser, C.; Klimo, K.; Nieger, M.; Maier, A.; Fiebig, H.-H.; Atodiresei, I.; Raabe, G.; Fleischhauer, J. Potential cancer chemopreventive in vitro activities of monomeric xanthone derivatives from the marine algicolous fungus Monodictys putredinis. J. Nat. Prod. 2007, 70, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Tsuchida, Y.; Mizoue, K.; Hanada, K. NG-011 AND NG-012, novel potentiators of nerve growth factor II. The structure determination of NG-011 AND NG-012. J. Antibiot. 1992, 45, 1566–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, S.-y.; Yang, C.-l.; Chen, R.-x.; Wu, M.-s.; Li, C.; Fu, S.-b.; Liu, J.-g. III Type Polyketone Compounds Produced by Streptomyces sp. FJS 31-2 from the Soil of Fanjing Mountain. Nat. Prod. Res. Dev. 2018, 30, 1160. [Google Scholar]
- Lu, X.; Xu, N.; Dai, H.-F.; Mei, W.-L.; Yang, Z.-X.; Pei, Y.-H. Three new compounds from endophytic fungus L10 of Cephalotaxus hainanensis. J. Asian Nat. Prod. Res. 2009, 11, 397–400. [Google Scholar] [CrossRef]






| 1 (Acetone-d6) | 2 (CDCl3) | |||
|---|---|---|---|---|
| No. | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) |
| 1 | 163.8, C | 160.0, C | ||
| 2 | 110.6, CH | 6.54 (d, 8.2) | 105.3, CH | 6.54 (d, 8.1) |
| 3 | 140.2, CH | 7.47 (t, 8.2) | 136.1, CH | 7.42 (t, 8.1) |
| 4 | 110.5, C | 6.48 (d, 8.2) | 108.0, CH | 6.58 (d, 8.1) |
| 5 | 160.0, C | 155.2, C | ||
| 6 | 94.3, C | 81.8, C | ||
| 7 | 204.5, C | 72.2, CH | 4.42 (d, 2.1) | |
| 8 | 38.3, CH | 3.12 (m) | 25.3, CH | 2.35 (m) |
| 9 | 39.5, CH2 | 2.14 (m) | 27.1, CH2 | 1.73 (m) |
| 2.09 (m) | ||||
| 10 | 67.8, CH | 4.61 (br t, 2.5) | 65.8, CH | 4.53 (s) |
| 11 | 79.6, C | 71.9, C | ||
| 12 | 196.9, C | 193.2, C | ||
| 13 | 108.4, C | 105.0, C | ||
| 14 | 63.5, CH2 | 4.83 (d, 13.1) | 58.1, CH2 | 3.88 (d, 13.5) |
| 3.96 (d, 13.1) | 4.20 (d, 13.5) | |||
| 15 | 15.4, CH3 | 1.08 (d, 6.4) | 15.1, CH3 | 1.17 (d, 6.7) |
| 1-OH | 10.8 (s) | |||
| 3 | 4 | |||
|---|---|---|---|---|
| No. | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) |
| 1 | 100.3, CH | 6.61 (d, 2.2) | ||
| 2 | 167.5, C | 164.9, C | ||
| 3 | 105.1, CH | 7.34 (d, 1.9) | 110.5, C | |
| 4 | 138.7, C | 166.4, C | ||
| 5 | 99.9, C | 121.7, C | ||
| 6 | 165.8, C | 146.4, CH | 7.48 (s) | |
| 7 | 112.2, C | 66.0, CH | 4.63 (m) | |
| 8 | 153.7, C | 39.4, CH2 | 1.70 (m) | |
| 9 | 165.7, C | 18.7, CH2 | 1.50 (m) | |
| 10 | 139.3, C | 13.2, CH3 | 0.92 (t, 7.4) | |
| 11 | 119.5, CH | 7.00 (m) | 9.8, CH3 | 1.99 (s) |
| 12 | 158.7, C | 60.7, CH3 | 3.95 (s) | |
| 13 | 103.8, CH | 7.00 (m) | ||
| 14 | 25.7, CH3 | 2.85 (s) | ||
| 15 | 56.3, CH3 | 3.99 (s) | ||
| 1’ | 101.5, CH | 5.80 (d, 4.3) | ||
| 2’ | 73.1, CH | 4.31 (t, 5.2) | ||
| 3’ | 88.2, CH | 4.20 (m) | ||
| 4’ | 70.8, CH | 4.20 (m) | ||
| 5’ | 62.9, CH2 | 3.71 (m) | ||
| Compounds | MIC (μg/mL) |
|---|---|
| Bacillus subtilis | |
| 1 | >100 |
| 2 | >100 |
| 3 | 16 |
| 4 | >100 |
| Streptomycin sulfate | 64 |
| Compounds | Growth Inhibition IC50 (µg/mL) Values | |
|---|---|---|
| Hela | MCF-7 | |
| 1 | 5.92 ± 0.04 | 7.50 ± 0.02 |
| 2 | >20 | >20 |
| 3 | >20 | >20 |
| 4 | >20 | >20 |
| Colchicine | 0.36 ± 0.07 | 0.44 ± 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, B.; Li, Y.; Wei, W.; Liu, G.; Xiao, C.; He, B.; Zhang, C.; Rajput, N.A.; Ye, Y.; Yan, W. Chemical Investigation of Endophytic Diaporthe unshiuensis YSP3 Reveals New Antibacterial and Cytotoxic Agents. J. Fungi 2023, 9, 136. https://doi.org/10.3390/jof9020136
Khan B, Li Y, Wei W, Liu G, Xiao C, He B, Zhang C, Rajput NA, Ye Y, Yan W. Chemical Investigation of Endophytic Diaporthe unshiuensis YSP3 Reveals New Antibacterial and Cytotoxic Agents. Journal of Fungi. 2023; 9(2):136. https://doi.org/10.3390/jof9020136
Chicago/Turabian StyleKhan, Babar, Yu Li, Wei Wei, Guiyou Liu, Cheng Xiao, Bo He, Chen Zhang, Nasir Ahmed Rajput, Yonghao Ye, and Wei Yan. 2023. "Chemical Investigation of Endophytic Diaporthe unshiuensis YSP3 Reveals New Antibacterial and Cytotoxic Agents" Journal of Fungi 9, no. 2: 136. https://doi.org/10.3390/jof9020136