Frailty as a Predictor of In-Hospital Outcome in Patients with Myocardial Infarction
Abstract
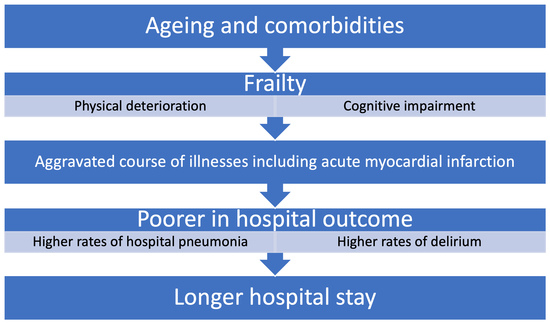
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nishihira, K.; Yoshioka, G.; Kuriyama, N.; Ogata, K.; Kimura, T.; Matsuura, H.; Furugen, M.; Koiwaya, H.; Watanabe, N.; Shibata, Y. Impact of frailty on outcomes in elderly patients with acute myocardial infarction who undergo percutaneous coronary intervention. Eur. Heart J. Qual. Care Clin. Outcomes 2021, 7, 189–197. [Google Scholar] [CrossRef] [PubMed]
- García-Blas, S.; Bonanad, C.; Fernández-Cisnal, A.; Sastre-Arbona, C.; Ruescas-Nicolau, M.A.; González D’Gregorio, J.; Valero, E.; Miñana, G.; Palau, P.; Tarazona-Santabalbina, F.J.; et al. Frailty Scales for Prognosis Assessment of Older Adult Patients after Acute Myocardial Infarction. J. Clin. Med. 2021, 10, 4278. [Google Scholar] [CrossRef]
- Ekerstad, N.; Pettersson, S.; Alexander, K.; Andersson, D.; Eriksson, S.; Janzon, M.; Lindenberger, M.; Swahn, E.; Alfredsson, J. Frailty as an instrument for evaluation of elderly patients with non-ST-segment elevation myocardial infarction: A follow-up after more than 5 years. Eur. J. Prev. Cardiol. 2018, 25, 1813–1821. [Google Scholar] [CrossRef]
- Patel, A.; Goodman, S.G.; Yan, A.T.; Alexander, K.P.; Wong, C.L.; Cheema, A.N.; Udell, J.A.; Kaul, P.; D’Souza, M.; Hyun, K.; et al. Frailty and Outcomes After Myocardial Infarction: Insights From the Concordance Registry. J. Am. Heart Assoc. 2018, 7, e009859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodkinson, H.M. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1972, 1, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.; Downs, T.D.; Cash, H.R.; Grotz, R.C. Progress in development of the index of ADL. Gerontologist 1970, 10, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Cudmore, S.; Robertson, S.; Stephen, J.; Haga, K.; Weir, C.J.; Murray, S.A.; Boyd, K.; Gunn, J.; Iqbal, J.; et al. Frailty assessment and risk prediction by GRACE score in older patients with acute myocardial infarction. BMC Geriatr. 2020, 20, 102. [Google Scholar] [CrossRef] [PubMed]
- Borovac, J.A.; Mohamed, M.O.; Kontopantelis, E.; Alkhouli, M.; Alraies, M.C.; Cheng, R.K.; Elgendy, I.Y.; Velagapudi, P.; Paul, T.K.; Van Spall, H.G.C.; et al. Frailty Among Patients With Acute ST-Elevation Myocardial Infarction in the United States: The Impact of the Primary Percutaneous Coronary Intervention on In-Hospital Outcomes. J. Invasive Cardiol. 2022, 34, E55–E64. [Google Scholar] [PubMed]
- Damluji, A.A.; Huang, J.; Bandeen-Roche, K.; Forman, D.E.; Gerstenblith, G.; Moscucci, M.; Resar, J.R.; Varadhan, R.; Walston, J.D.; Segal, J.B. Frailty Among Older Adults With Acute Myocardial Infarction and Outcomes From Percutaneous Coronary Interventions. J. Am. Heart Assoc. 2019, 8, e013686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curcio, F.; Gerundo, G.; Sasso, G.; Panicara, V.; Liguori, I.; Testa, G.; Della-Morte, D.; Gargiulo, G.; Galizia, G.; Ungar, A.; et al. Type 2 myocardial infarction: Is it a geriatric syndrome? Aging Clin. Exp. Res. 2020, 32, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Putthapiban, P.; Vutthikraivit, W.; Rattanawong, P.; Sukhumthammarat, W.; Kanjanahattakij, N.; Kewcharoen, J.; Amanullah, A. Association of frailty with all-cause mortality and bleeding among elderly patients with acute myocardial infarction: A systematic review and meta-analysis. J. Geriatr. Cardiol. 2020, 17, 270–278. [Google Scholar] [PubMed]
- Kleczynski, P.; Dziewierz, A.; Bagienski, M.; Rzeszutko, L.; Sorysz, D.; Trebacz, J.; Sobczynski, R.; Tomala, M.; Stapor, M.; Dudek, D. Impact of frailty on mortality after transcatheter aortic valve implantation. Am. Heart J. 2017, 185, 52–58. [Google Scholar] [CrossRef]
- Iwai-Saito, K.; Shobugawa, Y.; Aida, J.; Kondo, K. Frailty is associated with susceptibility and severity of pneumonia in older adults (A JAGES multilevel cross-sectional study). Sci. Rep. 2021, 11, 7966. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.H.; Zhou, G.D.; Wang, J.F. Delirium after primary percutaneous coronary intervention in aged individuals with acute ST-segment elevation myocardial infarction: A retrospective study. Exp. Ther. Med. 2019, 17, 3807–3813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, S.; Gonuguntala, K.; Rojulpote, C.; Kumar, M.; Corradi, J.P.; Chen, K. Delirium is an important predictor of mortality in elderly patients with ST-elevation myocardial infarction: Insight from National Inpatient Sample database. Coron. Artery Dis. 2020, 31, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Özbek, K.; Hasbek, E.; Koç, F. Delirium due to contrast toxicity after coronary angioplasty. Anadolu Kardiyol. Derg. 2012, 12, 612. [Google Scholar] [PubMed] [Green Version]

| Age (years) | 82 ± 5 |
| Male gender (%) | 56 |
| ST-elevation MI (%) | 24 |
| Non-ST-elevation MI (%) | 76 |
| Diabetes mellitus (%) | 38 |
| Arterial hypertension (%) | 93 |
| Smoking (%) | 27 |
| Chronic obstructive pulmonary disease (%) | 9 |
| History of PCI (%) | 27 |
| History of MI (%) | 29 |
| History of CABG (%) | 7 |
| History of stroke (%) | 9 |
| LVEF (Q1; Q3) | 45 (30; 50) |
| BMI (kg/m2) | 27.5 ± 4 |
| BSA (m2) | 1.84 ± 0.18 |
| Hemoglobin (g/dL) | 12.9 ± 1.65 |
| White blood count (103/µL) | 10.8 ± 3.9 |
| Platelet count (103/µL) | 239 ± 81 |
| Glomerular filtration rate (ml/min/1.73 m2) | 60.3 ± 18.9 |
| Abbreviated Mental Test Score (Q1; Q3) | 10 (8, 10) |
| Activity of Daily Living (Q1; Q3) | 6 (6, 6) |
| Instrumental Activity of Daily Living (Q1; Q3) | 23 (19, 24) |
| Clinical Frailty Scale (Q1; Q3) | 3 (3, 4) |
| Coronary angiography (%) | 98 |
| Treatment with PCI (%) | 72 |
| Treatment with CABG (%) | 4 |
| ASA (%) | 96 |
| Clopidogrel (%) | 74 |
| Ticagrelor (%) | 18 |
| Prasugrel (%) | 0 |
| B-blocker (%) | 94 |
| Angiotensin converting enzyme inhibitor (%) | 87 |
| Statin (%) | 96 |
| Mortality (%) | 2 |
| Stroke (%) | 0 |
| Red blood cell transfusion (%) | 2 |
| Delirium (%) | 9 |
| Hospital acquired pneumonia (%) | 24 |
| Length of hospital stay (days) (Q1; Q3) | 8 (7; 12) |
| AUC | p | |
|---|---|---|
| Delirium | ||
| Activity of Daily Living | 0.76 | 0.052 |
| Instrumental Activity of Daily Living | 0.81 | 0.023 |
| Clinical Frailty Scale | 0.86 | 0.009 |
| Abbreviated Mental Test Score | 0.78 | 0.04 |
| Pneumonia | ||
| Activity of Daily Living | 0.49 | 0.94 |
| Instrumental Activity of Daily Living | 0.67 | 0.065 |
| Clinical Frailty Scale | 0.62 | 0.19 |
| Abbreviated Mental Test Score | 0.69 | 0.036 |
| Unadjusted Analysis | Analysis Adjusted for Age, Gender and Type of MI | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Delirium | ||||||
| Activity of Daily Living | 0.45 | 0.23–0.88 | 0.02 | 0.32 | 0.12–0.85 | 0.022 |
| Instrumental Activity of Daily Living | 0.8 | 0.65–0.97 | 0.023 | 0.75 | 0.58–0.96 | 0.025 |
| Clinical Frailty Scale | 2.63 | 1.23–5.6 | 0.012 | 6.4 | 1.49–27.4 | 0.013 |
| Abbreviated Mental Test Score | 0.59 | 0.4–0.88 | 0.009 | 0.57 | 0.36–0.88 | 0.013 |
| Pneumonia | ||||||
| Activity of Daily Living | 1.19 | 0.56–2.56 | 0.65 | 0.92 | 0.39–2.17 | 0.85 |
| Instrumental Activity of Daily Living | 0.89 | 0.78–1.02 | 0.11 | 0.8 | 0.66–0.97 | 0.021 |
| Clinical Frailty Scale | 1.35 | 0.84–2.16 | 0.21 | 2.7 | 1.23–5.9 | 0.013 |
| Abbreviated Mental Test Score | 0.77 | 0.57–1.04 | 0.08 | 0.75 | 0.55–1.03 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Węgiel, M.; Kleczyński, P.; Dziewierz, A.; Rzeszutko, Ł.; Surdacki, A.; Bartuś, S.; Rakowski, T. Frailty as a Predictor of In-Hospital Outcome in Patients with Myocardial Infarction. J. Cardiovasc. Dev. Dis. 2022, 9, 145. https://doi.org/10.3390/jcdd9050145
Węgiel M, Kleczyński P, Dziewierz A, Rzeszutko Ł, Surdacki A, Bartuś S, Rakowski T. Frailty as a Predictor of In-Hospital Outcome in Patients with Myocardial Infarction. Journal of Cardiovascular Development and Disease. 2022; 9(5):145. https://doi.org/10.3390/jcdd9050145
Chicago/Turabian StyleWęgiel, Michał, Paweł Kleczyński, Artur Dziewierz, Łukasz Rzeszutko, Andrzej Surdacki, Stanisław Bartuś, and Tomasz Rakowski. 2022. "Frailty as a Predictor of In-Hospital Outcome in Patients with Myocardial Infarction" Journal of Cardiovascular Development and Disease 9, no. 5: 145. https://doi.org/10.3390/jcdd9050145