Respiratory-Induced Amplitude Modulation of Forcecardiography Signals
Abstract
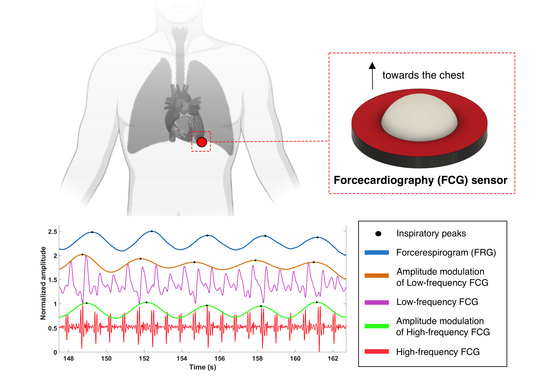
:1. Introduction
2. Materials and Methods
2.1. Sensor Placement and Measurement Setup
2.2. Signal Processing
2.3. Statistical Analyses
3. Results
3.1. Morphological Comparison
3.2. Statistical Analyses
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robinson, T.F.; Factor, S.M.; Sonnenblick, E.H. The Heart as a Suction Pump. Sci. Am. 1986, 254, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Krasny, R.; Kammermeier, H.; Köhler, J. Biomechanics of valvular plane displacement of the heart. Basic Res. Cardiol. 1991, 86, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, D.; Burcher, M.; Declerck, J.; Noble, J.A. Respiratory motion compensation for 3-D freehand echocardiography. Ultrasound Med. Biol. 2001, 27, 1615–1620. [Google Scholar] [CrossRef]
- Wang, Y.; Riederer, S.J.; Ehman, R.L. Respiratory motion of the heart: Kinematics and the implications for the spatial resolution in coronary imaging. Magn. Reson. Med. 1995, 33, 713–719. [Google Scholar] [CrossRef]
- Benchimol, A.; Dimond, E.G. The Apex Cardiogram In Ischaemic Heart Disease. Heart 1962, 24, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, A.; Dimond, E.G. The normal and abnormal apexcardiogram. Its physiologic variation and its relation to intracardiac events. Am. J. Cardiol. 1963, 12, 368–381. [Google Scholar] [CrossRef]
- Zanetti, J.; Salerno, D. Seismocardiography: A new technique for recording cardiac vibrations. Concept, method, and initial observations. J. Cardiovasc. Technol. 1990, 9, 111–118. [Google Scholar]
- Inan, O.T.; Migeotte, P.F.; Park, K.S.; Etemadi, M.; Tavakolian, K.; Casanella, R.; Zanetti, J.; Tank, J.; Funtova, I.; Prisk, G.K.; et al. Ballistocardiography and Seismocardiography: A Review of Recent Advances. IEEE J. Biomed. Health Inform. 2015, 19, 1414–1427. [Google Scholar] [CrossRef]
- Taebi, A.; Solar, B.E.; Bomar, A.J.; Sandler, R.H.; Mansy, H.A. Recent Advances in Seismocardiography. Vibration 2019, 2, 64–86. [Google Scholar] [CrossRef]
- Lin, W.; Chou, W.; Chang, P.; Chou, C.; Wen, M.; Ho, M.; Lee, M. Identification of Location Specific Feature Points in a Cardiac Cycle Using a Novel Seismocardiogram Spectrum System. IEEE J. Biomed. Health Inform. 2018, 22, 442–449. [Google Scholar] [CrossRef]
- Sørensen, K.; Schmidt, S.E.; Jensen, A.S.; Søgaard, P.; Struijk, J.J. Definition of Fiducial Points in the Normal Seismocardiogram. Sci. Rep. 2018, 8, 15455. [Google Scholar] [CrossRef] [Green Version]
- Sloan, A.W. Phonocardiography. Glasgow. Med. J. 1951, 32, 159–174. [Google Scholar] [PubMed]
- Giordano, N.; Knaflitz, M. A Novel Method for Measuring the Timing of Heart Sound Components through Digital Phonocardiography. Sensors 2019, 19, 1868. [Google Scholar] [CrossRef]
- Jafari Tadi, M.; Lehtonen, E.; Saraste, A.; Tuominen, J.; Koskinen, J.; Teräs, M.; Airaksinen, J.; Pänkäälä, M.; Koivisto, T. Gyrocardiography: A New Non-invasive Monitoring Method for the Assessment of Cardiac Mechanics and the Estimation of Hemodynamic Variables. Sci. Rep. 2017, 7, 6823. [Google Scholar] [CrossRef] [PubMed]
- Sieciński, S.; Kostka, P.S.; Tkacz, E.J. Gyrocardiography: A Review of the Definition, History, Waveform Description, and Applications. Sensors 2020, 20, 6675. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, Y.; Skoric, J.; Xu, S.; Roche, P.J.R.; Lortie, M.; Gagnon, S.; Plant, D.V. Real-Time Cardiac Beat Detection and Heart Rate Monitoring from Combined Seismocardiography and Gyrocardiography. Sensors 2019, 19, 3472. [Google Scholar] [CrossRef] [PubMed]
- Hossein, A.; Rabineau, J.; Gorlier, D.; Del Rio, J.I.J.; van de Borne, P.; Migeotte, P.F.; Nonclercq, A. Kinocardiography Derived from Ballistocardiography and Seismocardiography Shows High Repeatability in Healthy Subjects. Sensors 2021, 21, 815. [Google Scholar] [CrossRef] [PubMed]
- Munck, K.; Sørensen, K.; Struijk, J.J.; Schmidt, S.E. Multichannel seismocardiography: An imaging modality for investigating heart vibrations. Physiol. Meas. 2020, 41, 115001. [Google Scholar] [CrossRef]
- Dehkordi, P.; Khosrow-Khavar, F.; Di Rienzo, M.; Inan, O.T.; Schmidt, S.E.; Blaber, A.P.; Sørensen, K.; Struijk, J.J.; Zakeri, V.; Lombardi, P.; et al. Comparison of Different Methods for Estimating Cardiac Timings: A Comprehensive Multimodal Echocardiography Investigation. Front. Physiol. 2019, 10, 1057. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, E.; Fratini, A.; Esposito, D.; Naik, G.; Polley, C.; Gargiulo, G.D.; Bifulco, P. Forcecardiography: A Novel Technique to Measure Heart Mechanical Vibrations onto the Chest Wall. Sensors 2020, 20, 3885. [Google Scholar] [CrossRef]
- Andreozzi, E.; Centracchio, J.; Punzo, V.; Esposito, D.; Polley, C.; Gargiulo, G.D.; Bifulco, P. Respiration Monitoring via Forcecardiography Sensors. Sensors 2021, 21, 3996. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, E.; Gargiulo, G.D.; Esposito, D.; Bifulco, P. A Novel Broadband Forcecardiography Sensor for Simultaneous Monitoring of Respiration, Infrasonic Cardiac Vibrations and Heart Sounds. Front. Physiol. 2021, 12, 725716. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.; Andreozzi, E.; Fratini, A.; Gargiulo, G.; Savino, S.; Niola, V.; Bifulco, P. A Piezoresistive Sensor to Measure Muscle Contraction and Mechanomyography. Sensors 2018, 18, 2553. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.; Andreozzi, E.; Gargiulo, G.D.; Fratini, A.; D’Addio, G.; Naik, G.R.; Bifulco, P. A Piezoresistive Array Armband With Reduced Number of Sensors for Hand Gesture Recognition. Front. Neurorobot. 2020, 13, 114. [Google Scholar] [CrossRef]
- Esposito, D.; Gargiulo, G.D.; Parajuli, N.; Cesarelli, G.; Andreozzi, E.; Bifulco, P. Measurement of muscle contraction timing for prosthesis control: A comparison between electromyography and force-myography. In Proceedings of the 2020 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Bari, Italy, 1 June–1 July 2020. [Google Scholar] [CrossRef]
- Andreozzi, E.; Centracchio, J.; Esposito, D.; Bifulco, P. A Comparison of Heart Pulsations Provided by Forcecardiography and Double Integration of Seismocardiogram. Bioengineering 2022, 9, 167. [Google Scholar] [CrossRef]
- Centracchio, J.; Andreozzi, E.; Esposito, D.; Gargiulo, G.D.; Bifulco, P. Detection of Aortic Valve Opening and Estimation of Pre-Ejection Period in Forcecardiography Recordings. Bioengineering 2022, 9, 89. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Ran, K. Bland-Altman and Correlation Plot. MATLAB Central File Exchange. 2020. Available online: https://www.mathworks.com/matlabcentral/fileexchange/45049-bland-altman-and-correlation-plot (accessed on 28 May 2020).
- Pandia, K.; Inan, O.T.; Kovacs, G.T.; Giovangrandi, L. Extracting respiratory information from seismocardiogram signals acquired on the chest using a miniature accelerometer. Physiol. Meas. 2012, 33, 1643–1660. [Google Scholar] [CrossRef]
- Kozia, C.; Herzallah, R. Advanced Fusion and Empirical Mode Decomposition-Based Filtering Methods for Breathing Rate Estimation from Seismocardiogram Signals. Information 2021, 12, 368. [Google Scholar] [CrossRef]
- Solar, B.E.; Taebi, A.; Mansy, H.A. Classification of seismocardiographic cycles into lung volume phases. In Proceedings of the 2017 IEEE Signal Processing in Medicine and Biology Symposium (SPMB), Philadelphia, PA, USA, 2 December 2017. [Google Scholar] [CrossRef]
- Amit, G.; Shukha, K.; Gavriely, N.; Intrator, N. Respiratory modulation of heart sound morphology. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H796–H805. [Google Scholar] [CrossRef]
- Ishikawa, K.; Tamura, T. Study of respiratory influence on the intensity of heart sound in normal subjects. Angiology 1979, 30, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Gao, J.; Ruan, C.; Qiu, T.; Park, Y. Modeling of heart sound morphology and analysis of the morphological variations induced by respiration. Comput. Biol. Med. 2013, 43, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Azevedo Coste, C.; Nierat, M.C.; Renaux, S.; Similowski, T.; Guiraud, D. Respiratory Monitoring Based on Tracheal Sounds: Continuous Time-Frequency Processing of the Phonospirogram Combined with Phonocardiogram-Derived Respiration. Sensors 2020, 21, 99. [Google Scholar] [CrossRef] [PubMed]










| Respiratory Acts | Missed Acts | Spurious Acts | |||||
|---|---|---|---|---|---|---|---|
| Subject | FRG | LF-FCG | HF-FCG | LF-FCG | HF-FCG | LF-FCG | HF-FCG |
| #1 | 57 | 58 | 57 | 4 | 0 | 5 | 0 |
| #2 | 21 | 28 | 21 | 3 | 0 | 10 | 0 |
| #3 | 83 | 86 | 83 | 0 | 1 | 3 | 1 |
| #4 | 52 | 53 | 52 | 2 | 0 | 3 | 0 |
| #5 | 18 | 18 | 18 | 0 | 0 | 0 | 0 |
| #6 | 41 | 41 | 40 | 0 | 1 | 0 | 0 |
| Total | 272 | 284 | 271 | 9 | 2 | 21 | 1 |
| Sensitivity (%) | PPV (%) | |
|---|---|---|
| LF-FCG | 96.7 | 92.6 |
| HF-FCG | 99.3 | 99.6 |
| Slope | Intercept (s) | R2 | Bias | p-Value | LoA (s) | |
|---|---|---|---|---|---|---|
| LF-FCG | 1.05 | −0.147 | 0.86 | Non-significant | 0.65 | ±1.34 |
| HF-FCG | 0.991 | 0.0350 | 0.95 | Non-significant | 0.91 | ±0.710 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Centracchio, J.; Andreozzi, E.; Esposito, D.; Gargiulo, G.D. Respiratory-Induced Amplitude Modulation of Forcecardiography Signals. Bioengineering 2022, 9, 444. https://doi.org/10.3390/bioengineering9090444
Centracchio J, Andreozzi E, Esposito D, Gargiulo GD. Respiratory-Induced Amplitude Modulation of Forcecardiography Signals. Bioengineering. 2022; 9(9):444. https://doi.org/10.3390/bioengineering9090444
Chicago/Turabian StyleCentracchio, Jessica, Emilio Andreozzi, Daniele Esposito, and Gaetano D. Gargiulo. 2022. "Respiratory-Induced Amplitude Modulation of Forcecardiography Signals" Bioengineering 9, no. 9: 444. https://doi.org/10.3390/bioengineering9090444