Engineering Smooth Muscle to Understand Extracellular Matrix Remodeling and Vascular Disease
Abstract
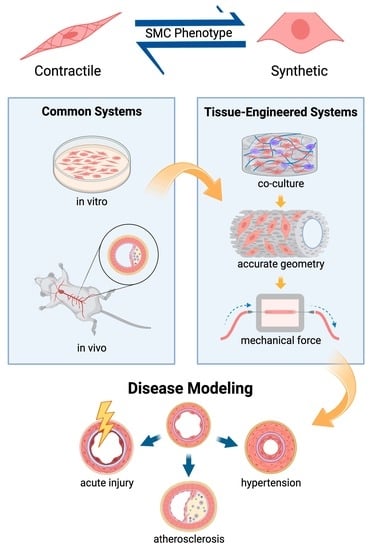
:1. Introduction
2. ECM Properties Affecting SMC Phenotype
2.1. Stiffness
2.2. Fibrillar Protein Composition
2.3. Non-Fibrillar Proteins and Matrix Modifiers
3. Engineering Complex In Vitro Models of Smooth Muscle
3.1. Mechanical Stimulation in Culture Systems
3.2. Organ-on-a-Chip and Multicellular Systems
3.3. Tissue Engineered Vascular Grafts and Pulsatile flow
4. Engineering of Smooth Muscle to Elucidate Mechanisms of Vascular Disease
4.1. Hypertension, Pulmonary Arterial Hypertension, and Atherosclerosis
4.2. Insult and Acute Injury
4.3. Genetic Mutations
5. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Andersson, C.; Vasan, R.S. Epidemiology of cardiovascular disease in young individuals. Nat. Rev. Cardiol. 2017, 15, 230–240. [Google Scholar] [CrossRef]
- Ma, Z.; Mao, C.; Jia, Y.; Fu, Y.; Kong, W. Extracellular matrix dynamics in vascular remodeling. Cell Physiol. 2020, 319, C481–C499. [Google Scholar] [CrossRef]
- Savoji, H.; Mohammadi, M.H.; Rafatian, N.; Toroghi, M.K.; Wang, E.Y.; Zhao, Y.; Korolj, A.; Ahadian, S.; Radisic, M. Cardiovascular disease models: A game changing paradigm in drug discovery and screening. Biomaterials 2020, 198, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Milani-Nejad, N.; Janssen, P.M.L. Small and large animal models in cardiac contraction research: Advantages and disadvantages. Pharmacol. Ther. 2014, 141, 235–249. [Google Scholar] [CrossRef]
- Tsang, H.G.; Rashdan, N.A.; Whitelaw, C.B.A.; Corcoran, B.M.; Summers, K.M.; MacRae, V.E. Large animal models of cardiovascular disease. Cell Biochem. Funct. 2016, 34, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-H.G.; Rumma, R.T.; Ozaki, C.K.; Edelman, E.R.; Chen, C.S. Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise. Cell Stem Cell 2018, 22, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular Regulation of Vascular Smooth Muscle Cell Differentiation in Development and Disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Ding, X.; Song, Y.; Chen, W.; Liang, J.; Yang, L.; Fan, Y.; Li, S.; Zhou, Y. Matrix stiffness regulates SMC functions via TGF-β signaling pathway. Biomaterials 2019, 221, 119407. [Google Scholar] [CrossRef]
- Jeong, K.; Kim, J.H.; Murphy, J.M.; Park, H.; Kim, S.J.; Rodriguez, Y.A.; Kong, H.; Choi, C.; Guan, J.-L.; Taylor, J.M.; et al. Nuclear Focal Adhesion Kinase Controls Vascular Smooth Muscle Cell Proliferation and Neointimal Hyperplasia Through GATA4-Mediated Cyclin D1 Transcription. Circ. Res. 2019, 125, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Johnson, R.; Sharma, S.; Ding, X.; Bryant, S.J.; Tan, W. Tethering transforming growth factor β1 to soft hydrogels guides vascular smooth muscle commitment from human mesenchymal stem cells. Acta Biomater. 2020, 105, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Sanyour, H.; Remund, T.; Kelly, P.; Hong, Z. Vascular extracellular matrix and fibroblasts-coculture directed differentiation of human mesenchymal stem cells toward smooth muscle-like cells for vascular tissue engineering. Mater. Sci. Eng. C 2018, 93, 61–69. [Google Scholar] [CrossRef]
- Eoh, J.H.; Shen, N.; Burke, J.A.; Hinderer, S.; Xia, Z.; Schenke-Layland, K.; Gerecht, S. Enhanced elastin synthesis and maturation in human vascular smooth muscle tissue derived from induced-pluripotent stem cells. Acta Biomater. 2017, 52, 49–59. [Google Scholar] [CrossRef]
- Daley, M.C.; Bonzanni, M.; MacKenzie, A.M.; Kaplan, D.L.; Black, L.D., III. The effects of membrane potential and extracellular matrix composition on vascular differentiation of cardiac progenitor cells. Biochem. Biophys. Res. Commun. 2020, 530, 240–245. [Google Scholar] [CrossRef]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved Patch-Clamp Techniques for High-Resolution Current Recording from Cells and Cell-Free Membrane Patches. Pflug. Arch. 1981, 391, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Bobi, J.; Garabito, M.; Solanes, N.; Cidad, P.; Ramos-Pérez, V.; Ponce, A.; Rigol, M.; Freixa, X.; Pérez-Martínez, C.; de Prado, A.P.; et al. Kv1.3 blockade inhibits proliferation of vascular smooth muscle cells in vitro and intimal hyperplasia in vivo. Transl. Res. 2020, 224, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Steppan, J.; Wang, H.; Bergman, Y.; Rauer, M.J.; Tan, S.; Jandu, S.; Nandakumar, K.; Barreto-Ortiz, S.; Cole, R.N.; Boronina, T.N.; et al. Lysyl oxidase-like 2 depletion is protective in age-associated vascular stiffening. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H49–H59. [Google Scholar] [CrossRef]
- Kim, S.K.; McCurley, A.T.; DuPont, J.J.; Aronovitz, M.; Moss, M.E.; Stillman, I.E.; Karumanchi, S.A.; Christou, D.D.; Jaffe, I.Z. Smooth Muscle Cell–Mineralocorticoid Receptor as a Mediator of Cardiovascular Stiffness With Aging. Hypertension 2018, 71, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Kiss, T.; Nyúl-Tóth, Á.; Gulej, R.; Tarantini, S.; Csipo, T.; Mukli, P.; Ungvari, A.; Balasubramanian, P.; Yabluchanskiy, A.; Benyo, Z.; et al. Old blood from heterochronic parabionts accelerates vascular aging in young mice: Transcriptomic signature of pathologic smooth muscle remodeling. GeroScience 2022, 44, 953–981. [Google Scholar] [CrossRef]
- Mui, K.L.; Bae, Y.H.; Gao, L.; Liu, S.-L.; Xu, T.; Radice, G.L.; Chen, C.S.; Assoian, R.K. N-Cadherin Induction by ECM Stiffness and FAK Overrides the Spreading Requirement for Proliferation of Vascular Smooth Muscle Cells. Cell Rep. 2015, 10, 1477–1486. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Wang, Y.-X.; Ren, K.-F.; Wang, Y.-B.; Fu, G.-S.; Jia, J. The influence of substrate stiffness on osteogenesis of vascular smooth muscle cells. Colloids Surf. B Biointerfaces 2021, 197, 111388. [Google Scholar] [CrossRef]
- Floren, M.; Bonani, W.; Dharmarajan, A.; Mott, A.; Migliaresi, C.; Tan, W. Human mesenchymal stem cells cultured on silk hydrogels with variable stiffness and growth factor differentiate into mature smooth muscle cell phenotype. Acta Biomater. 2016, 31, 156–166. [Google Scholar] [CrossRef]
- Xie, S.-A.; Zhang, T.; Wang, J.; Zhao, F.; Zhang, Y.-P.; Yao, W.-J.; Hur, S.S.; Yeh, Y.-T.; Pang, W.; Zheng, L.-S.; et al. Matrix stiffness determines the phenotype of vascular smooth muscle cell in vitro and in vivo: Role of DNA methyltransferase 1. Biomaterials 2018, 155, 203–216. [Google Scholar] [CrossRef]
- Petit, C.; Yousefi, A.-A.K.; Moussa, O.B.; Michel, J.-B.; Guignandon, A.; Avril, S. Regulation of SMC traction forces in human aortic thoracic aneurysms. Biomech. Modeling Mechanobiol. 2021, 20, 717–731. [Google Scholar] [CrossRef]
- Pezzoli, D.; Paolo, J.D.; Kumra, H.; Fois, G.; Candiani, G.; Reinhardt, D.P.; Mantovani, D. Fibronectin promotes elastin deposition, elasticity and mechanical strength in cellularised collagen-based scaffolds. Biomaterials 2018, 180, 130–142. [Google Scholar] [CrossRef]
- Ryan, A.J.; O'Brien, F.J. Insoluble elastin reduces collagen scaffold stiffness, improves viscoelastic properties, and induces a contractile phenotype in smooth muscle cells. Biomaterials 2015, 73, 296–307. [Google Scholar] [CrossRef]
- Rickel, A.P.; Sanyour, H.J.; Leyda, N.A.; Hong, Z. Extracellular Matrix Proteins and Substrate Stiffness Synergistically Regulate Vascular Smooth Muscle Cell Migration and Cortical Cytoskeleton Organization. ACS Appl. Biomater. 2020, 3, 2360–2369. [Google Scholar] [CrossRef] [PubMed]
- Senatus, L.M.; Schmidt, A.M. The AGE-RAGE Axis: Implications for Age-Associated Arterial Diseases. Front. Genet. 2017, 8, 187. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Wu, J.; Zhang, L.; Yang, P.; Tu, Q.; Huang, N. Tailoring of the titanium surface by preparing cardiovascular endothelial extracellular matrix layer on the hyaluronic acid micro-pattern for improving biocompatibility. Colloids Surf. B Biointerfaces 2015, 128, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Wu, J.; Reho, J.J.; Lu, K.-T.; Brozoski, D.T.; Kumar, G.; Werthman, A.M.; Sebastiao Donato Silva, J.; Veitia, P.C.M.; Wackman, K.K.; et al. RhoBTB1 reverses established arterial stiffness in angiotensin II–induced hypertension by promoting actin depolymerization. JCI Insight 2022, 7, e158043. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Wagenseil, J. Elastic fibers and biomechanics of the aorta: Insights from mouse studies. Matrix Biol. 2020, 85–86, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.L.; Beck, H.C.; Irmukhamedov, A.; Jensen, P.S.; Olsen, M.H.; Rasmussen, L.M. Proteome Analysis of Human Arterial Tissue Discloses Associations Between the Vascular Content of Small Leucine-Rich Repeat Proteoglycans and Pulse Wave Velocity. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Jover, E.; Silvente, A.; Marin, F.; Martinez-Gonzalez, J.; Orriols, M.; Martinez, C.M.; Puche, C.M.; Valdés, M.; Rodriguez, C.; Hernández-Romero, D. Inhibition of enzymes involved in collagen cross-linking reduces vascular smooth muscle cell calcification. FASEB J. 2018, 32, 4459–4469. [Google Scholar] [CrossRef]
- Dhar, S.; Sun, Z.; Meininger, G.A.; Hill, M.A. Nonenzymatic glycation interferes with fibronectin-integrin interactions in vascular smooth muscle cells. Microcirculation 2017, 24, e12347. [Google Scholar] [CrossRef] [PubMed]
- Horita, H.; Wysoczynski, C.L.; Walker, L.A.; Moulton, K.S.; Li, M.; Ostriker, A.; Tucker, R.; McKinsey, T.A.; Churchill, M.E.A.; Nemenoff, R.A.; et al. Nuclear PTEN functions as an essential regulator of SRF-dependent transcription to control smooth muscle differentiation. Nat. Commun. 2016, 7, 10830. [Google Scholar] [CrossRef]
- Liu, S.-L.; Bae, Y.H.; Yu, C.; Monslow, J.; Hawthorne, E.A.; Castagnino, P.; Branchetti, E.; Ferrari, G.; Damrauer, S.M.; Puré, E.; et al. Matrix metalloproteinase-12 is an essential mediator of acute and chronic arterial stiffening. Sci. Rep. 2015, 5, 17189. [Google Scholar] [CrossRef]
- Lim, H.Y.; Lim, S.Y.; Tan, C.K.; Thiam, C.H.; Goh, C.C.; Carbajo, D.; Chew, S.H.S.; See, P.; Chakarov, S.; Wang, X.N.; et al. Hyaluronan Receptor LYVE-1-Expressing Macrophages Maintain Arterial Tone through Hyaluronan-Mediated Regulation of Smooth Muscle Cell Collagen. Immunity 2018, 49, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Schiller, H.B.; Friedel, C.C.; Boulegue, C.; Fässler, R. Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep. 2011, 12, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Chan, X.Y.; Volkova, E.; Eoh, J.; Black, R.; Fang, L.; Gorashi, R.; Song, J.; Wang, J.; Elliott, M.B.; Barreto-Ortiz, S.F.; et al. HIF2A gain-of-function mutation modulates the stiffness of smooth muscle cells and compromises vascular mechanics. IScience 2021, 24, 102246. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, M.G.; Gardner, W.S.; Bennett, L.; Sather, B.A.; Yanagisawa, H.; Wagenseil, J.E. The Effects of Elastic Fiber Protein Insufficiency and Treatment on the Modulus of Arterial Smooth Muscle Cells. J. Biomech. Eng. 2014, 136, 021030. [Google Scholar] [CrossRef]
- Beamish, J.A.; He, P.; Kottke-Marchant, K.; Marchant, R.E. Molecular Regulation of Contractile Smooth Muscle Cell Phenotype: Implications for Vascular Tissue Engineering. Tissue Eng. Part B Rev. 2010, 16, 467–491. [Google Scholar] [CrossRef] [Green Version]
- Ramaswamy, A.K.; Vorp, D.A.; Weinbaum, J.S. Functional Vascular Tissue Engineering Inspired by Matricellular Proteins. Front. Cardiovasc. Med. 2019, 6, 74. [Google Scholar] [CrossRef]
- Mantella, L.-E.; Quan, A.; Verma, S. Variability in vascular smooth muscle cell stretch-induced responses in 2D culture. Vasc. Cell 2015, 7, 7. [Google Scholar] [CrossRef]
- Jensen, L.F.; Bentzon, J.F.; Albarrán-Juárez, J. The Phenotypic Responses of Vascular Smooth Muscle Cells Exposed to Mechanical Cues. Cells 2021, 10, 2209. [Google Scholar] [CrossRef]
- Walters, B.; Turner, P.A.; Rolauffs, B.; Hart, M.L.; Stegemann, J.P. Controlled Growth Factor Delivery and Cyclic Stretch Induces a Smooth Muscle Cell-like Phenotype in Adipose-Derived Stem Cells. Cells 2021, 10, 3123. [Google Scholar] [CrossRef]
- Sato, K.; Nitta, M.; Ogawa, A. A Microfluidic Cell Stretch Device to Investigate the Effects of Stretching Stress on Artery Smooth Muscle Cell Proliferation in Pulmonary Arterial Hypertension. Inventions 2019, 4, 1. [Google Scholar] [CrossRef]
- Yamashiro, Y.; Thang, B.Q.; Ramirez, K.; Shin, S.J.; Kohata, T.; Ohata, S.; Nguyen, T.A.V.; Ohtsuki, S.; Nagayama, K.; Yanagisawa, H. Matrix mechanotransduction mediated by thrombospondin-1/integrin/YAP in the vascular remodeling. Proc. Natl. Acad. Sci. USA 2020, 117, 9896–9905. [Google Scholar] [CrossRef]
- Lévesque, L.; Loy, C.; Lainé, A.; Drouin, B.; Chevallier, P.; Mantovani, D. Incrementing the Frequency of Dynamic Strain on SMC-Cellularised Collagen-Based Scaffolds Affects Extracellular Matrix Remodeling and Mechanical Properties. ACS Biomater. Sci. Eng. 2018, 4, 3759–3767. [Google Scholar] [CrossRef] [PubMed]
- Bono, N.; Pezzoli, D.; Levesque, L.; Loy, C.; Candiani, G.; Fiore, G.B.; Mantovani, D. Unraveling the role of mechanical stimulation on smooth muscle cells: A comparative study between 2D and 3D models. Biotechnol. Bioeng. 2016, 113, 2254–2263. [Google Scholar] [CrossRef]
- Shi, Z.-D.; Tarbell, J.M. Fluid Flow Mechanotransduction in Vascular Smooth Muscle Cells and Fibroblasts. Ann. Biomed. Eng. 2011, 39, 1608–1619. [Google Scholar] [CrossRef] [PubMed]
- Hiroshima, Y.; Oyama, Y.; Sawasaki, K.; Nakamura, M.; Kimura, N.; Kawahito, K.; Fujie, H.; Sakamoto, N. A Compressed Collagen Construct for Studying Endothelial–Smooth Muscle Cell Interaction Under High Shear Stress. Ann. Biomed. Eng. 2022, 50, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, A.; Albers, H.J.; Passiera, R.; Mummery, C.L.; Berg, A.v.d.; Orlova, V.V.; Meer, A.D.v.d. Advanced in vitro models of vascular biology: Human induced pluripotent stem cells and organ-on-chip technology. Adv. Drug Deliv. Rev. 2019, 140, 68–77. [Google Scholar] [CrossRef]
- Cuenca, M.V.; Cochrane, A.; Hil, F.E.v.d.; Vries, A.A.F.d.; Oberstein, S.A.J.L.; Mummery, C.L.; Orlova, V.V. Engineered 3D vessel-on-chip using hiPSC-derived endothelial- and vascular smooth muscle cells. Stem Cell Rep. 2021, 16, 2159–2168. [Google Scholar] [CrossRef]
- Cho, M.; Park, J.-K. Fabrication of a Perfusable 3D In Vitro Artery-Mimicking Multichannel System for Artery Disease Models. ACS Biomater. Sci. Eng. 2020, 6, 5326–5336. [Google Scholar] [CrossRef]
- Yasotharan, S.; Pinto, S.; Sled, J.G.; Bolzef, S.-S.; Günther, A. Artery-on-a-chip platform for automated, multimodal assessment of cerebral blood vessel structure and function. Lab A Chip 2015, 15, 2660–2669. [Google Scholar] [CrossRef]
- Barreto-Ortiz, S.F.; Fradkin, J.; Eoh, J.; Trivero, J.; Davenport, M.; Ginn, B.; Mao, H.-Q.; Gerecht, S. Fabrication of 3-dimensional multicellular microvascular structures. FASEB J. 2015, 29, 3302–3314. [Google Scholar] [CrossRef]
- Zhang, X.; Battiston, K.G.; Labow, R.S.; Simmons, C.A.; Santerre, J.P. Generating favorable growth factor and protease release profiles to enable extracellular matrix accumulation within an in vitro tissue engineering environment. Acta Biomater. 2017, 54, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, A.K.; Sides, R.E.; Cunnane, E.M.; Lorentz, K.L.; Reines, L.M.; Vorp, D.A.; Weinbaum, J.S. Adipose-derived stromal cell secreted factors induce the elastogenesis cascade within 3D aortic smooth muscle cell constructs. Matrix Biol. Plus 2019, 4, 100014. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Shi, Y.; Fan, Z.; Zhou, Y.; Song, Y.; Liu, Y.; Yu, G.; An, Q.; Zhu, W. Inflammatory Smooth Muscle Cells Induce Endothelial Cell Alterations to Influence Cerebral Aneurysm Progression via Regulation of Integrin and VEGF Expression. Cell Transplant. 2018, 28, 713–722. [Google Scholar] [CrossRef]
- Naegeli, K.M.; Kural, M.H.; Li, Y.; Wang, J.; Hugentobler, E.A.; Niklason, L.E. Bioengineering Human Tissues and the Future of Vascular Replacement. Circ. Res. 2022, 131, 109–126. [Google Scholar] [CrossRef]
- Luo, J.; Qin, L.; Zhao, L.; Gui, L.; Ellis, M.; Huang, Y.; Kural, M.; Clark, J.A.; Ono, S.; Wang, J.; et al. Tissue-Engineered Vascular Grafts with Advanced Mechanical Strength from Human iPSCs. Cell Stem Cell 2020, 26, 251–261. [Google Scholar] [CrossRef]
- Yalcin, I.; Horakova, J.; Mikes, P.; Sadikoglu, T.G.; Domin, R.; Lukas, D. Design of Polycaprolactone Vascular Grafts. J. Ind. Text. 2014, 45, 813–833. [Google Scholar] [CrossRef]
- Leal, B.B.J.; Wakabayashi, N.; Oyama, K.; Kamiya, H.; Braghirolli, D.I.; Pranke, P. Vascular Tissue Engineering: Polymers and Methodologies for Small Caliber Vascular Grafts. Front. Cardiovasc. Med. 2021, 7, 592361. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-X.; Li, L.; Zhou, S.-Y.; Cao, J.-H.; Liang, W.-H.; Tian, Y.; Shi, X.-T.; Yang, X.-B.; Wu, D.-Y. A biomimetic orthogonal-bilayer tubular scaffold for the co-culture of endothelial cells and smooth muscle cells. RSC Adv. 2021, 11, 31783–31790. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.C.; Pagani, R.; Vallet-Regí, M.; Peña, J.; Rámila, A.; Izquierdo, I.; Portolés, M.T. In vitro biocompatibility assessment of poly(ε-caprolactone) films using L929 mouse fibroblasts. Biomaterials 2004, 25, 5603–5611. [Google Scholar] [CrossRef] [PubMed]
- Ardila, D.C.; Tamimi, E.; Doetschman, T.; Wagner, W.R.; Geest, J.P.V. Modulating smooth muscle cell response by the release of TGFβ2 from tubular scaffolds for vascular tissue engineering. J. Control. Release 2019, 299, 44–52. [Google Scholar] [CrossRef]
- Qin, K.; Wang, F.; Simpson, R.M.L.; Zheng, X.; Wang, H.; Hu, Y.; Gao, Z.; Xu, Q.; Zhao, Q. Hyaluronan promotes the regeneration of vascular smooth muscle with potent contractile function in rapidly biodegradable vascular grafts. Biomaterials 2020, 257, 120226. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.M.; Ahn, H.; Arenas-Herrera, J.; Kim, C.; Abolbashari, M.; Atala, A.; Yoo, J.J.; Lee, S.J. Electrospun vascular scaffold for cellularized small diameter blood vessels: A preclinical large animal study. Acta Biomater. 2017, 59, 58–67. [Google Scholar] [CrossRef]
- Dash, B.C.; Setia, O.; Gorecka, J.; Peyvandi, H.; Duan, K.; Lopes, L.; Nie, J.; Berthiaume, F.; Dardik, A.; Hsia, H.C. A Dense Fibrillar Collagen Scaffold Differentially Modulates Secretory Function of iPSC-Derived Vascular Smooth Muscle Cells to Promote Wound Healing. Cells 2020, 9, 966. [Google Scholar] [CrossRef]
- Nakayama, K.H.; Joshi, P.A.; Lai, E.S.; Gujar, P.; Joubert, L.-M.; Chen, B.; Huang, N.F. Bilayered vascular graft derived from human induced pluripotent stem cells with biomimetic structure and function. Future Med. 2015, 10, 745–755. [Google Scholar] [CrossRef]
- Bono, N.; Meghezi, S.; Soncini, M.; Piola, M.; Mantovani, D.; Fiore, G.B. A Dual-Mode Bioreactor System for Tissue Engineered Vascular Models. Ann. Biomed. Eng. 2017, 45, 1496–1510. [Google Scholar] [CrossRef]
- Chimene, D.; Peak, C.W.; Gentry, J.L.; Carrow, J.K.; Cross, L.M.; Mondragon, E.; Cardoso, G.B.; Kaunas, R.; Gaharwar, A.K. Nanoengineered Ionic–Covalent Entanglement (NICE) Bioinks for 3D Bioprinting. ACS Appl. Mater. Interfaces 2018, 10, 9957–9968. [Google Scholar] [CrossRef] [PubMed]
- Schöneberg, J.; De Lorenzi, F.; Theek, B.; Blaeser, A.; Rommel, D.; Kuehne, A.J.; Kießling, F.; Fischer, H. Engineering biofunctional in vitro vessel models using a multilayer bioprinting technique. Sci. Rep. 2018, 8, 10430. [Google Scholar] [CrossRef]
- Cao, X.; Maharjan, S.; Ashfaq, R.; Yu, J.S.; Zhang, S. Bioprinting of Small-Diameter Blood Vessels. Engineering 2021, 7, 832–844. [Google Scholar] [CrossRef]
- Gao, G.; Kim, H.; Kim, B.S.; Kong, J.S.; Lee, J.Y.; Park, B.W.; Chae, S.; Kim, J.; Ban, K.; Jang, J.; et al. Tissue-engineering of vascular grafts containing endothelium and smooth-muscle using triple-coaxial cell printing featured. Appl. Phys. Rev. 2019, 6, 041402. [Google Scholar] [CrossRef]
- Dash, B.C.; Levi, K.; Schwan, J.; Luo, J.; Bartulos, O.; Wu, H.; Qiu, C.; Yi, T.; Ren, Y.; Campbell, S.; et al. Tissue-Engineered Vascular Rings from Human iPSC-Derived Smooth Muscle Cells. Stem Cell Rep. 2016, 7, 19–28. [Google Scholar] [CrossRef]
- Marzia, J.; Brauchle, E.M.; Schenke-Layland, K.; Rolle, M.W. Non-invasive functional molecular phenotyping of human smooth muscle cells utilized in cardiovascular tissue engineering. Acta Biomater. 2019, 89, 193–205. [Google Scholar] [CrossRef]
- Stenmark, K.R.; Frid, M.G.; Graham, B.B.; Tuder, R.M. Dynamic and diverse changes in the functional properties of vascular smooth muscle cells in pulmonary hypertension. Cardiovasc. Res. 2018, 114, 551–564. [Google Scholar] [CrossRef]
- Harvey, A.; Montezano, A.C.; Lopes, R.A.; Rios, F.; Touyz, R.M. Vascular Fibrosis in Aging and Hypertension: Molecular Mechanisms and Clinical Implications. Can. J. Cardiol. 2016, 32, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Guignabert, C.; Tu, L.; Girerd, B.; Ricard, N.; Huertas, A.; Montani, D.; Humbert, M. New Molecular Targets of Pulmonary Vascular Remodeling in Pulmonary Arterial Hypertension: Importance of Endothelial Communication. Chest 2015, 147, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Lehoux, S.; Esposito, B.; Merval, R.; Tedgui, A. Differential Regulation of Vascular Focal Adhesion Kinase by Steady Stretch and Pulsatility. Circulation 2005, 111, 643–649. [Google Scholar] [CrossRef] [Green Version]
- Al-Hilal, T.A.; Keshavarz, A.; Kadry, H.; Lahooti, B.; Al-Obaida, A.; Ding, Z.; Li, W.; Kamm, R.; McMurtry, I.F.; Lahm, T.; et al. Pulmonary-arterial-hypertension (PAH)-on-a-chip: Fabrication, validation and application. Lab A Chip 2020, 20, 3334–3345. [Google Scholar] [CrossRef]
- Seidman, M.A.; Mitchell, R.N.; Stone, J.R. Pathophysiology of Atherosclerosis. In Cellular and Molecular Pathobiology of Cardiovascular Disease; Monte, S., Willis, J.W.H., Stone, J.R., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2014; pp. 221–237. [Google Scholar] [CrossRef]
- Hosseini, V.; Mallone, A.; Mirkhani, N.; Noir, J.; Salek, M.; Pasqualini, F.S.; Schuerle, S.; Khademhosseini, A.; Hoerstrup, S.P.; Vogel, V. A Pulsatile Flow System to Engineer Aneurysm and Atherosclerosis Mimetic Extracellular Matrix. Adv. Sci. 2020, 7, 2000173. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, J.D.; Goettsch, C.; Bertazzo, S.; Maldonado, N.; Ruiz, J.L.; Goh, W.; Yabusaki, K.; Faits, T.; Bouten, C.; Franck, G.; et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat. Mater. 2016, 15, 335–343. [Google Scholar] [CrossRef]
- Kural, M.H.; Dai, G.; Niklason, L.E.; Gui, L. An Ex Vivo Vessel Injury Model to Study Remodeling. Cell Transplant. 2018, 27, 1375–1389. [Google Scholar] [CrossRef] [PubMed]
- Staiculescu, M.C.; Ramirez-Perez, F.I.; Castorena-Gonzalez, J.A.; Hong, Z.; Sun, Z.; Meininger, G.A.; Martinez-Lemus, L.A. Lysophosphatidic acid induces integrin activation in vascular smooth muscle and alters arteriolar myogenic vasoconstriction. Front. Physiol. 2014, 5, 413. [Google Scholar] [CrossRef]
- Lacolley, P.; Regnault, V.; Segers, P.; Laurent, S. Vascular Smooth Muscle Cells and Arterial Stiffening: Relevance in Development, Aging, and Disease. Physiol. Rev. 2017, 97, 1555–1617. [Google Scholar] [CrossRef]
- Bogunovic, N.; Meekel, J.P.; Majolée, J.; Hekhuis, M.; Pyszkowski, J.; Jockenhövel, S.; Kruse, M.; Riesebos, E.; Micha, D.; Blankensteijn, J.D.; et al. Patient-Specific 3-Dimensional Model of Smooth Muscle Cell and Extracellular Matrix Dysfunction for the Study of Aortic Aneurysms. J. Endovasc. Ther. 2021, 28, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Atchison, L.; Zhang, H.; Cao, K.; Truskey, G.A. A Tissue Engineered Blood Vessel Model of Hutchinson-Gilford Progeria Syndrome Using Human iPSC-derived Smooth Muscle Cells. Sci. Rep. 2017, 7, 8168. [Google Scholar] [CrossRef]
- Granata, A.; Serrano, F.; Bernard, W.G.; McNamara, M.; Low, L.; Sastry, P.; Sinha, S. An iPSC-derived vascular model of Marfan syndrome identifies key mediators of smooth muscle cell death. Nat. Genet. 2016, 49, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Qin, L.; Park, J.; Kural, M.H.; Huang, Y.; Shi, X.; Riaz, M.; Wang, J.; Ellis, M.W.; Anderson, C.W.; et al. Readily Available Tissue-Engineered Vascular Grafts Derived From Human Induced Pluripotent Stem Cells. Circ. Res. 2022, 130, 925–927. [Google Scholar] [CrossRef]
- Lashkarinia, S.S.; Coban, G.; Kose, B.; Salihoglu, E.; Pekkan, K. Computational modeling of vascular growth in patient-specific pulmonary arterial patch reconstructions. J. Biomech. 2021, 117, 110274. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.J.; Jayendiran, R.; Farzaneh, S.; Campisi, S.; Viallon, M.; Croisille, P.; Avril, S. Coupling hemodynamics with mechanobiology in patient-specific computational models of ascending thoracic aortic aneurysms. Comput. Methods Programs Biomed. 2021, 205, 106107. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.M.; Zbinden, J.C.; Ramachandra, A.B.; Lindsey, S.E.; Szafron, J.M.; Reinhardt, J.W.; Heitkemper, M.; Best, C.A.; Mirhaidari, G.J.M.; Chang, Y.-C.; et al. Tissue engineered vascular grafts transform into autologous neovessels capable of native function and growth. Commun. Med. 2022, 2, 3. [Google Scholar] [CrossRef] [PubMed]


| Extracellular Matrix (ECM) Property | Factor or Pathway of Interest | Experimental Model | Findings |
|---|---|---|---|
| Stiffness | Lysyl oxidase-like 2 (LOXL2) | Human smooth muscle cells (SMCs) in 2D culture; LOXL2 knockout mice |
|
| Mineralocorticoid receptor (MR) | MR deleted male mice |
| |
| Circulating molecules | Parabiosis with young and old mice |
| |
| Focal adhesion kinases (FAKs) and N-cadherin | FAK knockout mice |
| |
| FAK and N-cadherin knockout mice |
| ||
| Transforming factor beta (TGF-β) signaling pathway | Human SMCs on collagen I (COL1)-coated polyacrylamide (PA) gels |
| |
| Human SMCs on polymethylsiloxane (PDMS) |
| ||
| Human SMCs on silk fibroin gels |
| ||
| DNA methyltransferase I (DNMT1) | Human SMCs on fibronectin (FN)-coated PA gels; acute aortic injury and chronic kidney failure mouse models |
| |
| Ascending thoracic aortic aneurysm | Healthy and aneurysmal human SMCs on compliant hydrogels |
| |
| Fibrillar Protein Composition | Fibronectin | Porcine SMCs suspended in COL1-FN gels |
|
| Elastin | Human smooth muscle cells on porous collagen-elastin scaffold sheets |
| |
| Collagen 1 and fibronectin | Human smooth muscle cells on ECM-coated polyacrylamide gels |
| |
| Non-fibrillar Protein Abundance and Structure | Advanced Glycation End products (AGEs) | Mice models |
|
| Hyaluronic acid (HA) | Human SMCs cultured on micropatterned and HA/ECM-coated titanium |
| |
| Rho-related BTB domain–containing protein 1 (RhoBTB1) | Angiotensin-II treated (hypertensive) mice |
| |
| Elastin assembly proteins | Knockout mice models |
| |
| Small leucine-rich repeat proteoglycans | Human coronary artery bypass patients |
| |
| Lysyl hydroxylase I (PLOD1), lysyl oxidase (LOX) | Human and mouse SMCs cultured in osteogenic medium |
| |
| Post-translationally modified (glycosylated) fibronectin (gFN) | Rat SMCs on fibronectin |
| |
| Protein and lipid phosphatase (PTEN) | Mice with SMC-specific-PTEN knockout; Isolated human atherosclerotic arteries |
| |
| Matrix metalloproteinase-12 (MMP12) | MMP12 knockout mice |
| |
| Matrixmetalloproteinase-9 (MMP9) | Macrophage depleted mice |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yarbrough, D.; Gerecht, S. Engineering Smooth Muscle to Understand Extracellular Matrix Remodeling and Vascular Disease. Bioengineering 2022, 9, 449. https://doi.org/10.3390/bioengineering9090449
Yarbrough D, Gerecht S. Engineering Smooth Muscle to Understand Extracellular Matrix Remodeling and Vascular Disease. Bioengineering. 2022; 9(9):449. https://doi.org/10.3390/bioengineering9090449
Chicago/Turabian StyleYarbrough, Danielle, and Sharon Gerecht. 2022. "Engineering Smooth Muscle to Understand Extracellular Matrix Remodeling and Vascular Disease" Bioengineering 9, no. 9: 449. https://doi.org/10.3390/bioengineering9090449