Managing the Heterogeneity of Mesenchymal Stem Cells for Cartilage Regenerative Therapy: A Review
Abstract
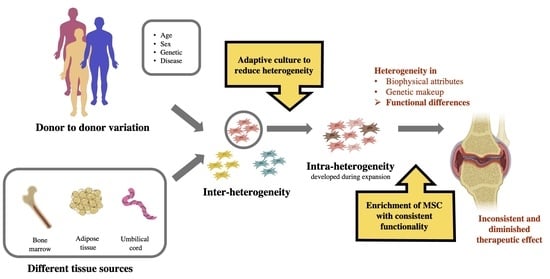
:1. Articular Cartilage Injury and Management
2. Mesenchymal Stem Cells (MSCs) for Cartilage Regeneration
3. Heterogeneity of MSCs
4. Managing the Heterogeneity of MSCs for More Effective Cartilage Regeneration
4.1. Reducing Expansion Period
4.2. Selecting MSCs Based on Specific Markers
| Specific MSC Markers | Results | Reference |
|---|---|---|
| CD73+ | High levels of COL2 and ACAN expression during chondrogenic differentiation, with stable levels of COL1, COLX, and MMP13. | [106] |
| CD105+ | Increased proliferation and improved colony formation. Enhanced chondrogenic potential in vitro with increased expression of SOX9, COL2, and ACAN. | [107,108] |
| CD271+ | Improved chondrogenic differentiation with a higher expression of Runx2 and COL2. Upregulation of genes associated with ECM production and cell adhesion; downregulation of genes associated with inflammation. Improved osteochondral defect healing while maintaining low angiogenesis in an athymic rat model. | [106,113,114] |
| CD146+ | Increased glycosaminoglycan production after chondrogenesis. Improved chondrogenic potential and cell migration. Chondroprotective effects during intra-articular implantation. Promoted long-term cartilage repair in a rat osteochondral defect model and demonstrated immunomodulation. | [117,118,119,120,121,122] |
| Stro-1+ | Increased proliferation and differentiation capacity. Increased immunosuppression and homing capabilities. | [124,125] |
| CD49f+ | Improved clonogenicity, adhesion, migration, and anti-apoptotic properties. | [127] |
| SSEA-4 | Improved growth and multipotency. Increased chondrogenicity. | [129,130,131] |
| GSTT1 | Homozygous negative GSTT1 MSCs demonstrate increased scalability and potency. | [53] |
4.3. Selecting MSCs Based on Specific Biophysical Attributes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | three-dimensional |
| ACI | autologous chondrocyte implantation |
| AD-MSC | adipose-derived mesenchymal stem cell |
| BM-MSC | bone marrow mesenchymal stem cell |
| ECM | extracellular matrix |
| EGF | epidermal growth factor |
| FACS | fluorescence-activated cell sorting |
| FGF2 | fibroblast growth factor 2 |
| GSTT1 | glutathione S-transferase theta 1 |
| IGF | insulin-like growth factor |
| M-ACI | matrix-induced autologous cartilage implantation |
| MACS | magnetic-activated cell sorting |
| MALBAC | multiple annealing and looping-based amplification cycles |
| MDA | multiple displacement amplification |
| MSC | mesenchymal stem cell |
| OATS | osteochondral autograft transfer system |
| PDGF-BB | platelet-derived growth factor-BB |
| scRNA-seq | single-cell RNA sequencing |
| SM-MSC | synovium membrane-derived mesenchymal stem cell |
| UCB-MSC | umbilical cord blood-derived mesenchymal stem cell |
References
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Greene, G.W.; Banquy, X.; Lee, D.W.; Lowrey, D.D.; Yu, J.; Israelachvili, J.N. Adaptive mechanically controlled lubrication mechanism found in articular joints. Proc. Natl. Acad. Sci. USA 2011, 108, 5255–5259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckwalter, J.; Mankin, H. Articular cartilage: Part I. J. Bone Jt. Surg. 1997, 79, 600. [Google Scholar] [CrossRef]
- Gomoll, A.H.; Minas, T. The quality of healing: Articular cartilage. Wound Repair Regen. 2014, 22, 30–38. [Google Scholar] [CrossRef]
- Morales, T.I. Chondrocyte moves: Clever strategies? Osteoarthr. Cartil. 2007, 15, 861–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckwalter, J.A.; Felson, D.T. Post-Traumatic Arthritis: Definitions and Burden of Disease. In Post-Traumatic Arthritis: Pathogenesis, Diagnosis and Management; Olson, M.D.S.A., Guilak, P.F., Eds.; Springer US: Boston, MA, USA, 2015; pp. 7–15. [Google Scholar]
- Buckwalter, J.A.; Mankin, H.J. Instructional Course Lectures, The American Academy of Orthopaedic Surgeons—Articular Cartilage. Part II: Degeneration and Osteoarthrosis, Repair, Regeneration, and Transplantation*†. JBJS 1997, 79, 612–632. [Google Scholar] [CrossRef]
- Gomoll, A.H.; Farr, J.; Gillogly, S.D.; Kercher, J.; Minas, T. Surgical Management of Articular Cartilage Defects of the Knee. JBJS 2010, 92, 2470–2490. [Google Scholar]
- Richter, W. Mesenchymal stem cells and cartilage in situ regeneration. J. Intern. Med. 2009, 266, 390–405. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, G.; Engebretsen, L.; Ludvigsen, T.C.; Drogset, J.O.; Grøntvedt, T.; Solheim, E.; Torbjørn, S.; Sally, R.; Vidar, I.; Oddmund, J. Autologous chondrocyte implantation compared with microfracture in the knee: A randomized trial. JBJS 2004, 86, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Olivos-Meza, A.; Madrazo-Ibarra, A.; de León, C.I.P. Arthroscopic Technique to Treat Articular Cartilage Lesions in the Patellofemoral Joint; IntechOpen: London, UK, 2018. [Google Scholar]
- Gudas, R.; Gudaitė, A.; Pocius, A.; Gudienė, A.; Čekanauskas, E.; Monastyreckienė, E.; Basevičius, A. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am. J. Sports Med. 2012, 40, 2499–2508. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, A.; Karnatzikos, G.; Kumar, A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1986–1996. [Google Scholar] [CrossRef]
- Orth, P.; Goebel, L.; Wolfram, U.; Ong, M.F.; Gräber, S.; Kohn, D.; Ignatius, A.; Pape, D.; Madry, H. Effect of subchondral drilling on the microarchitecture of subchondral bone: Analysis in a large animal model at 6 months. Am. J. Sports Med. 2012, 40, 828–836. [Google Scholar] [CrossRef]
- Richter, D.L.; Tanksley, J.A.; Miller, M.D. Osteochondral autograft transplantation: A review of the surgical technique and outcomes. Sports Med. Arthrosc. Rev. 2016, 24, 74–78. [Google Scholar] [CrossRef]
- Bedi, A.; Feeley, B.T.; Williams, R.J., III. Management of Articular Cartilage Defects of the Knee. JBJS 2010, 92, 994–1009. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, R. Particulated juvenile articular cartilage allograft transplantation for osteochondral lesions of the talus. Foot Ankle Clin. 2013, 18, 79–87. [Google Scholar] [CrossRef]
- Niemeyer, P.; Albrecht, D.; Andereya, S.; Angele, P.; Ateschrang, A.; Aurich, M.; Baumann, M.; Bosch, U.; Erggelet, C.; Fickert, S.; et al. Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: A guideline by the working group “Clinical Tissue Regeneration” of the German Society of Orthopaedics and Trauma (DGOU). Knee 2016, 23, 426–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhosale, A.M.; Richardson, J.B. Articular cartilage: Structure, injuries and review of management. Br. Med. Bull. 2008, 87, 77–95. [Google Scholar] [CrossRef]
- Zheng, M.-H.; Willers, C.; Kirilak, L.; Yates, P.; Xu, J.; Wood, D.; Shimmin, A. Matrix-induced autologous chondrocyte implantation (MACI®): Biological and histological assessment. Tissue Eng. 2007, 13, 737–746. [Google Scholar] [CrossRef]
- Caron, M.M.J.; Emans, P.J.; Coolsen, M.M.E.; Voss, L.; Surtel, D.A.M.; Cremers, A.; van Rhijn, L.W.; Welting, T.J.M. Redifferentiation of dedifferentiated human articular chondrocytes: Comparison of 2D and 3D cultures. Osteoarthr. Cartil. 2012, 20, 1170–1178. [Google Scholar] [CrossRef] [Green Version]
- Saris, D.; Price, A.; Widuchowski, W.; Bertrand-Marchand, M.; Caron, J.; Drogset, J.O.; Emans, P.; Podskubka, A.; Tsuchida, A.; Kili, S.; et al. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: Two-year follow-up of a prospective randomized trial. Am. J. Sports Med. 2014, 42, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Nazempour, A.; Van Wie, B.J. Chondrocytes, Mesenchymal Stem Cells, and Their Combination in Articular Cartilage Regenerative Medicine. Ann. Biomed. Eng. 2016, 44, 1325–1354. [Google Scholar] [CrossRef]
- Duan, L.; Ma, B.; Liang, Y.; Chen, J.; Zhu, W.; Li, M.; Wang, D. Cytokine networking of chondrocyte dedifferentiation in vitro and its implications for cell-based cartilage therapy. Am. J. Transl. Res. 2015, 7, 194–208. [Google Scholar]
- Reissis, D.; Tang, Q.O.; Cooper, N.C.; Carasco, C.F.; Gamie, Z.; Mantalaris, A.; Tsiridis, E. Current clinical evidence for the use of mesenchymal stem cells in articular cartilage repair. Expert Opin. Biol. Ther. 2016, 16, 535–557. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, H.; Muneta, T.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007, 327, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Hubka, K.M.; Dahlin, R.L.; Meretoja, V.V.; Kasper, F.K.; Mikos, A.G. Enhancing chondrogenic phenotype for cartilage tissue engineering: Monoculture and coculture of articular chondrocytes and mesenchymal stem cells. Tissue Eng. Part B Rev. 2014, 20, 641–654. [Google Scholar] [CrossRef] [Green Version]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Ma, J.; Han, J.; Zhang, W.; Ma, J. Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. Am. J. Transl. Res. 2019, 11, 6275–6289. [Google Scholar]
- Anderson, J.A.; Little, D.; Toth, A.P.; Moorman, C.T.; Tucker, B.S.; Ciccotti, M.G.; Guilak, T. Stem Cell Therapies for Knee Cartilage Repair: The Current Status of Preclinical and Clinical Studies. Am. J. Sports Med. 2013, 42, 2253–2261. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.-B.; Ha, C.-W.; Lee, C.-H.; Yoon, Y.C.; Park, Y.-G. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl. Med. 2016, 6, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Nishida, Y.; Takahashi, S.; Nakamura, H.; Mera, H.; Kashiwa, K.; Yoshiya, S.; Inagaki, Y.; Uematsu, K.; Tanaka, Y.; et al. Transplantation of autologous bone marrow-derived mesenchymal stem cells under arthroscopic surgery with microfracture versus microfracture alone for articular cartilage lesions in the knee: A multicenter prospective randomized control clinical trial. Regen. Ther. 2019, 11, 106–113. [Google Scholar] [CrossRef]
- Saris, T.F.F.; de Windt, T.S.; Kester, E.C.; Vonk, L.A.; Custers, R.J.H.; Saris, D.B.F. Five-Year Outcome of 1-Stage Cell-Based Cartilage Repair Using Recycled Autologous Chondrons and Allogenic Mesenchymal Stromal Cells: A First-in-Human Clinical Trial. Am. J. Sports Med. 2021, 49, 941–947. [Google Scholar] [CrossRef]
- Wu, L.; Prins, H.-J.; Helder, M.N.; van Blitterswijk, C.A.; Karperien, M. Trophic effects of mesenchymal stem cells in chondrocyte co-cultures are independent of culture conditions and cell sources. Tissue Eng. Part A 2012, 18, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.S.H.; Tjio, C.K.E.; Wong, J.R.Y.; Wong, K.L.; Chew, J.R.J.; Hui, J.H.P.; Toh, W.S. Mesenchymal stem cell exosomes for cartilage regeneration: A systematic review of preclinical in vivo studies. Tissue Eng. Part B Rev. 2021, 27, 1–13. [Google Scholar] [CrossRef]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef]
- Toh, W.S.; Lai, R.C.; Hui, J.H.P.; Lim, S.K. MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. Semin. Cell Dev. Biol. 2017, 67, 56–64. [Google Scholar] [CrossRef]
- Caplan, A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007, 213, 341–347. [Google Scholar] [CrossRef]
- Melief, S.M.; Schrama, E.; Brugman, M.H.; Tiemessen, M.M.; Hoogduijn, M.J.; Fibbe, W.E.; Roelofs, H. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells 2013, 31, 1980–1991. [Google Scholar] [CrossRef]
- Pers, Y.-M.; Maumus, M.; Bony, C.; Jorgensen, C.; Noël, D. Contribution of microRNAs to the immunosuppressive function of mesenchymal stem cells. Biochimie 2018, 155, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wong, K.L.; Ren, X.; Teo, K.Y.W.; Afizah, H.; Choo, A.B.H.; Lai, R.C.; Lim, S.K.; Hui, J.H.P.; Toh, W.S. Mesenchymal Stem Cell Exosomes Promote Functional Osteochondral Repair in a Clinically Relevant Porcine Model. Am. J. Sports Med. 2022, 50, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021, 14, 24. [Google Scholar] [CrossRef]
- Levy, O.; Kuai, R.; Siren, E.M.J.; Bhere, D.; Milton, Y.; Nissar, N.; De Biasio, M.; Heinelt, M.; Reeve, B.; Abdi, R.; et al. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 2020, 6, eaba6884. [Google Scholar] [CrossRef] [PubMed]
- Elahi, F.M.; Farwell, D.G.; Nolta, J.A.; Anderson, J.D. Preclinical translation of exosomes derived from mesenchymal stem/stromal cells. Stem Cells 2019, 38, 15–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Erickson, I.E.; Huang, A.H.; Garrity, S.T.; Mauck, R.L.; Steinberg, D.R. Donor Variation and Optimization of Human Mesenchymal Stem Cell Chondrogenesis in Hyaluronic Acid. Tissue Eng. Part A 2018, 24, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Zha, K.; Li, X.; Yang, Z.; Tian, G.; Sun, Z.; Sui, X.; Dai, Y.; Liu, S.; Guo, Q. Heterogeneity of mesenchymal stem cells in cartilage regeneration: From characterization to application. NPJ Regen. Med. 2021, 6, 14. [Google Scholar] [CrossRef]
- Kanawa, M.; Igarashi, A.; Ronald, V.S.; Higashi, Y.; Kurihara, H.; Sugiyama, M.; Saskianti, T.; Pan, H.; Kato, Y. Age-dependent decrease in the chondrogenic potential of human bone marrow mesenchymal stromal cells expanded with fibroblast growth factor-2. Cytotherapy 2013, 15, 1062–1072. [Google Scholar] [CrossRef]
- Siegel, G.; Kluba, T.; Hermanutz-Klein, U.; Bieback, K.; Northoff, H.; Schäfer, R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013, 11, 146. [Google Scholar] [CrossRef] [Green Version]
- Camernik, K.; Mihelič, A.; Mihalič, R.; Haring, G.; Herman, S.; Presen, D.M.; Janež, A.; Trebše, R.; Marc, J.; Zupan, J. Comprehensive analysis of skeletal muscle- and bone-derived mesenchymal stem/stromal cells in patients with osteoarthritis and femoral neck fracture. Stem Cell Res. Ther. 2020, 11, 146. [Google Scholar] [CrossRef]
- Murphy, J.M.; Dixon, K.; Beck, S.; Fabian, D.; Feldman, A.; Barry, F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002, 46, 704–713. [Google Scholar] [CrossRef]
- Sathiyanathan, P.; Samsonraj, R.M.; Tan, C.L.L.; Ling, L.; Lezhava, A.; Nurcombe, V.; Stanton, L.W.; Cool, S.M. A genomic biomarker that identifies human bone marrow-derived mesenchymal stem cells with high scalability. Stem Cells 2020, 38, 1124–1136. [Google Scholar] [CrossRef]
- Afizah, H.; Yang, Z.; Hui, J.H.; Ouyang, H.-W.; Lee, E.-H. A comparison between the chondrogenic potential of human bone marrow stem cells (BMSCs) and adipose-derived stem cells (ADSCs) taken from the same donors. Tissue Eng. 2007, 13, 659–666. [Google Scholar] [CrossRef]
- Hui, J.H.; Li, L.; Teo, Y.-H.; Ouyang, H.-W.; Lee, E.-H. Comparative study of the ability of mesenchymal stem cells derived from bone marrow, periosteum, and adipose tissue in treatment of partial growth arrest in rabbit. Tissue Eng. 2005, 11, 904–912. [Google Scholar] [CrossRef]
- Kohli, N.; Wright, K.T.; Sammons, R.L.; Jeys, L.; Snow, M.; Johnson, W.E.B. An In Vitro Comparison of the Incorporation, Growth, and Chondrogenic Potential of Human Bone Marrow versus Adipose Tissue Mesenchymal Stem Cells in Clinically Relevant Cell Scaffolds Used for Cartilage Repair. Cartilage 2015, 6, 252–263. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Wang, Y.; Zhao, C.; Guo, S.; Liu, S.; Jia, W.; Tuan, R.S.; Zhang, C. Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials 2012, 33, 7008–7018. [Google Scholar] [CrossRef]
- Peng, L.; Jia, Z.; Yin, X.; Zhang, X.; Liu, Y.; Chen, P.; Ma, K.; Zhou, C. Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev. 2008, 17, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Contentin, R.; Demoor, M.; Concari, M.; Desancé, M.; Audigié, F.; Branly, T.; Galéra, P. Comparison of the Chondrogenic Potential of Mesenchymal Stem Cells Derived from Bone Marrow and Umbilical Cord Blood Intended for Cartilage Tissue Engineering. Stem Cell Rev. Rep. 2020, 16, 126–143. [Google Scholar] [CrossRef]
- Fellows, C.R.; Matta, C.; Zakany, R.; Khan, I.M.; Mobasheri, A. Adipose, Bone Marrow and Synovial Joint-Derived Mesenchymal Stem Cells for Cartilage Repair. Front. Genet. 2016, 7, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, M.; Hildebrand, M.; Menzel, U.; Fahy, N.; Alini, M.; Lang, S.; Benneker, L.; Verrier, S.; Stoddart, M.J.; Bara, J.J. Phenotypic characterization of bone marrow mononuclear cells and derived stromal cell populations from human iliac crest, vertebral body and femoral head. Int. J. Mol. Sci. 2019, 20, 3454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivasubramaniyan, K.; Ilas, D.; Harichandan, A.; Bos, P.K.; Santos, D.L.; De Zwart, P.; Koevoet, W.J.; Owston, H.; Bühring, H.-J.; Jones, E.; et al. Bone Marrow–Harvesting Technique Influences Functional Heterogeneity of Mesenchymal Stem/Stromal Cells and Cartilage Regeneration. Am. J. Sports Med. 2018, 46, 3521–3531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.E.; Kim, S.J.; Yang, S.-J.; Joo, S.-Y.; Park, H.; Lee, K.W.; Yang, H.-M.; Park, J.B. Comparative characterization of mesenchymal stromal cells from multiple abdominal adipose tissues and enrichment of angiogenic ability via CD146 molecule. Cytotherapy 2017, 19, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Lopa, S.; Colombini, A.; Stanco, D.; De Girolamo, L.; Sansone, V.; Moretti, M. Donor-matched mesenchymal stem cells from knee infrapatellar and subcutaneous adipose tissue of osteoarthritic donors display differential chondrogenic and osteogenic commitment. Eur. Cell Mater. 2014, 27, 298–311. [Google Scholar] [PubMed]
- Wang, Z.; Chai, C.; Wang, R.; Feng, Y.; Huang, L.; Zhang, Y.; Xiao, X.; Yang, S.; Zhang, Y.; Zhang, X. Single-cell transcriptome atlas of human mesenchymal stem cells exploring cellular heterogeneity. Clin. Transl. Med. 2021, 11, e650. [Google Scholar] [CrossRef]
- Sun, C.; Wang, L.; Wang, H.; Huang, T.; Yao, W.; Li, J.; Zhang, X. Single-cell RNA-seq highlights heterogeneity in human primary Wharton’s jelly mesenchymal stem/stromal cells cultured in vitro. Stem Cell Res. Ther. 2020, 11, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ando, W.; Kutcher, J.J.; Krawetz, R.; Sen, A.; Nakamura, N.; Frank, C.B.; Hart, D.A. Clonal analysis of synovial fluid stem cells to characterize and identify stable mesenchymal stromal cell/mesenchymal progenitor cell phenotypes in a porcine model: A cell source with enhanced commitment to the chondrogenic lineage. Cytotherapy 2014, 16, 776–788. [Google Scholar] [CrossRef]
- Rennerfeldt, D.A.; Van Vliet, K.J. Concise Review: When Colonies Are Not Clones: Evidence and Implications of Intracolony Heterogeneity in Mesenchymal Stem Cells. Stem Cells 2016, 34, 1135–1141. [Google Scholar] [CrossRef] [Green Version]
- Cote, A.J.; McLeod, C.M.; Farrell, M.J.; McClanahan, P.D.; Dunagin, M.C.; Raj, A.; Mauck, R.L. Single-cell differences in matrix gene expression do not predict matrix deposition. Nat. Commun. 2016, 7, 10865. [Google Scholar] [CrossRef] [Green Version]
- Freeman, B.T.; Jung, J.P.; Ogle, B.M. Single-cell RNA-Seq of bone marrow-derived mesenchymal stem cells reveals unique profiles of lineage priming. PLoS ONE 2015, 10, e0136199. [Google Scholar] [CrossRef]
- Rennerfeldt, D.A.; Raminhos, J.S.; Leff, S.M.; Manning, P.; Van Vliet, K.J. Emergent heterogeneity in putative mesenchymal stem cell colonies: Single-cell time lapsed analysis. PLoS ONE 2019, 14, e0213452. [Google Scholar] [CrossRef] [Green Version]
- McLeod, C.; Mauck, R. On the origin and impact of mesenchymal stem cell heterogeneity: New insights and emerging tools for single cell analysis. Eur. Cells Mater. 2017, 34, 217. [Google Scholar] [CrossRef]
- Wilson, A.; Webster, A.; Genever, P. Nomenclature and heterogeneity: Consequences for the use of mesenchymal stem cells in regenerative medicine. Regen. Med. 2019, 14, 595–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, A.; Alegre-Aguarón, E.; O’Connell, G.; VandenBerg, C.; Aaron, R.; Vunjak-Novakovic, G.; Bulinski, J.C.; Ateshian, G.; Hung, C. Passage-dependent relationship between mesenchymal stem cell mobilization and chondrogenic potential. Osteoarthr. Cartil. 2015, 23, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Siennicka, K.; Zołocińska, A.; Dębski, T.; Pojda, Z. Comparison of the Donor Age-Dependent and In Vitro Culture-Dependent Mesenchymal Stem Cell Aging in Rat Model. Stem Cells Int. 2021, 2021, 6665358. [Google Scholar] [CrossRef] [PubMed]
- Jeske, R.; Yuan, X.; Fu, Q.; Bunnell, B.A.; Logan, T.M.; Li, Y. In vitro culture expansion shifts the immune phenotype of human adipose-derived mesenchymal stem cells. Front. Immunol. 2021, 12, 621744. [Google Scholar] [CrossRef]
- Solchaga, L.A.; Penick, K.; Porter, J.D.; Goldberg, V.M.; Caplan, A.I.; Welter, J.F. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J. Cell. Physiol. 2005, 203, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Solchaga, L.A.; Penick, K.; Goldberg, V.M.; Caplan, A.I.; Welter, J.F. Fibroblast growth factor-2 enhances proliferation and delays loss of chondrogenic potential in human adult bone-marrow-derived mesenchymal stem cells. Tissue Eng. Part A 2010, 16, 1009–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleary, M.A.; van Osch, G.J.; Brama, P.A.; Hellingman, C.A.; Narcisi, R. FGF, TGFβ and Wnt crosstalk: Embryonic to in vitro cartilage development from mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2015, 9, 332–342. [Google Scholar] [CrossRef]
- Choi, S.-C.; Kim, S.-J.; Choi, J.-H.; Park, C.-Y.; Shim, W.-J.; Lim, D.-S. Fibroblast growth factor-2 and-4 promote the proliferation of bone marrow mesenchymal stem cells by the activation of the PI3K-Akt and ERK1/2 signaling pathways. Stem Cells Dev. 2008, 17, 725–736. [Google Scholar] [CrossRef] [Green Version]
- Farré, J.; Farré, J.; Roura, S.; Farré, J.; Roura, S.; Prat-Vidal, C.; Hove-Madsen, L.; Cairo, J.J.; Godia, F.; Bragos, R.; et al. FGF-4 increases in vitro expansion rate of human adult bone marrow-derived mesenchymal stem cells. Growth Factors 2007, 25, 71–76. [Google Scholar] [CrossRef]
- Wijesinghe, S.J.; Ling, L.; Murali, S.; Qing, Y.H.; Hinkley, S.F.; Carnachan, S.M.; Bell, T.J.; Swaminathan, K.; Hui, J.H.; van Wijnen, A.J.; et al. Affinity selection of FGF2-binding heparan sulfates for ex vivo expansion of human mesenchymal stem cells. J. Cell. Physiol. 2017, 232, 566–575. [Google Scholar] [CrossRef]
- Titmarsh, D.M.; Tan, C.L.; Glass, N.R.; Nurcombe, V.; Cooper-White, J.J.; Cool, S.M. Microfluidic Screening Reveals Heparan Sulfate Enhances Human Mesenchymal Stem Cell Growth by Modulating Fibroblast Growth Factor-2 Transport. Stem Cells Transl Med. 2017, 6, 1178–1190. [Google Scholar] [CrossRef]
- Ling, L.; Ren, X.; Cao, X.; Hassan, A.B.M.; Mah, S.; Sathiyanathan, P.; Smith, R.A.A.; Tan, C.L.; Eio, M.; Samsonraj, R.M.; et al. Enhancing the Efficacy of Stem Cell Therapy with Glycosaminoglycans. Stem Cell Rep. 2020, 14, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Helledie, T.; Dombrowski, C.; Rai, B.; Lim, Z.X.; Hin, I.L.H.; Rider, D.A.; Stein, G.S.; Hong, W.; Van Wijnen, A.J.; Hui, J.H.; et al. Heparan sulfate enhances the self-renewal and therapeutic potential of mesenchymal stem cells from human adult bone marrow. Stem Cells Dev. 2012, 21, 1897–1910. [Google Scholar] [CrossRef] [PubMed]
- Gharibi, B.; Ghuman, M.S.; Hughes, F.J. Akt- and Erk-mediated regulation of proliferation and differentiation during PDGFRβ-induced MSC self-renewal. J. Cell Mol. Med. 2012, 16, 2789–2801. [Google Scholar] [CrossRef]
- Sun, X.; Gao, X.; Zhou, L.; Sun, L.; Lu, C. PDGF-BB-induced MT1-MMP expression regulates proliferation and invasion of mesenchymal stem cells in 3-dimensional collagen via MEK/ERK1/2 and PI3K/AKT signaling. Cell. Signal. 2013, 25, 1279–1287. [Google Scholar] [CrossRef]
- Lienemann, P.S.; Devaud, Y.R.; Reuten, R.; Simona, B.R.; Karlsson, M.; Weber, W.; Koch, M.; Lutolf, M.P.; Milleret, V.; Ehrbar, M. Locally controlling mesenchymal stem cell morphogenesis by 3D PDGF-BB gradients towards the establishment of an in vitro perivascular niche. Integr. Biol. 2014, 7, 101–111. [Google Scholar] [CrossRef]
- Grigorieva, O.; Arbatskiy, M.; Novoseletskaya, E.; Dyachkova, U.; Ishkin, A.; Kalinina, N.; Efimenko, A. Platelet-Derived Growth Factor Induces SASP-Associated Gene Expression in Human Multipotent Mesenchymal Stromal Cells but Does Not Promote Cell Senescence. Biomedicines 2021, 9, 1290. [Google Scholar] [CrossRef] [PubMed]
- Tamama, K.; Kawasaki, H.; Wells, A. Epidermal Growth Factor (EGF) Treatment on Multipotential Stromal Cells (MSCs). Possible Enhancement of Therapeutic Potential of MSC. J. Biomed. Biotechnol. 2010, 2010, 795385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, C.; James, S.; Kuntin, D.; Fox, J.; Newling, K.; Hollings, S.; Pennock, R.; Genever, P. Epidermal growth factor can signal via β-catenin to control proliferation of mesenchymal stem cells independently of canonical Wnt signalling. Cell. Signal. 2019, 53, 256–268. [Google Scholar] [CrossRef]
- Hu, C.; Wu, Y.; Wan, Y.; Wang, Q.; Song, J. Introduction of hIGF-1 Gene into Bone Marrow Stromal Cells and Its Effects on the Cell’s Biological Behaviors. Cell Transplant. 2008, 17, 1067–1081. [Google Scholar] [CrossRef] [Green Version]
- Youssef, A.; Han, V.K.M. Low Oxygen Tension Modulates the Insulin-Like Growth Factor-1 or -2 Signaling via Both Insulin-Like Growth Factor-1 Receptor and Insulin Receptor to Maintain Stem Cell Identity in Placental Mesenchymal Stem Cells. Endocrinology 2016, 157, 1163–1174. [Google Scholar] [CrossRef] [Green Version]
- Yeo, G.C.; Weiss, A.S. Soluble matrix protein is a potent modulator of mesenchymal stem cell performance. Proc. Natl. Acad. Sci. USA 2019, 116, 2042–2051. [Google Scholar] [CrossRef] [Green Version]
- Lindner, U.; Kramer, J.; Behrends, J.; Driller, B.; Wendler, N.-O.; Boehrnsen, F.; Rohwedel, J.; Schlenke, P. Improved proliferation and differentiation capacity of human mesenchymal stromal cells cultured with basement-membrane extracellular matrix proteins. Cytotherapy 2010, 12, 992–1005. [Google Scholar] [CrossRef]
- Somaiah, C.; Kumar, A.; Mawrie, D.; Sharma, A.; Patil, S.D.; Bhattacharyya, J.; Swaminathan, R.; Jaganathan, B.G. Collagen Promotes Higher Adhesion, Survival and Proliferation of Mesenchymal Stem Cells. PLoS ONE 2015, 10, e0145068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antebi, B.; Zhang, Z.; Wang, Y.; Lu, Z.; Chen, X.-D.; Ling, J. Stromal-cell-derived extracellular matrix promotes the proliferation and retains the osteogenic differentiation capacity of mesenchymal stem cells on three-dimensional scaffolds. Tissue Eng. Part C Methods 2015, 21, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Bas, G.; Loisate, S.; Hudon, S.F.; Woods, K.; Hayden, E.J.; Pu, X.; Beard, R.; Oxford, J.T.; Uzer, G. Low Intensity Vibrations Augment Mesenchymal Stem Cell Proliferation and Differentiation Capacity during in vitro Expansion. Sci. Rep. 2020, 10, 9369. [Google Scholar] [CrossRef] [PubMed]
- Touchstone, H.; Bryd, R.; Loisate, S.; Thompson, M.; Kim, S.; Puranam, K.; Senthilnathan, A.N.; Pu, X.; Beard, R.; Rubin, J.; et al. Recovery of stem cell proliferation by low intensity vibration under simulated microgravity requires LINC complex. NPJ Microgravity 2019, 5, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miskon, A.; Abdul Hamid, H.; Ramasamy, R.; Mohd Tawil, S. (Eds.) Enhanced proliferation potential of human umbilical cord mesenchymal stem cells through suspension induction and electromagnetic field exposure. In International Conference on the Development of Biomedical Engineering in Vietnam; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Hamid, H.A.; Sarmadi, V.H.; Prasad, V.; Ramasamy, R.; Miskon, A. Electromagnetic field exposure as a plausible approach to enhance the proliferation and differentiation of mesenchymal stem cells in clinically relevant scenarios. J. Zhejiang Univ. Sci. B. 2022, 23, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Bule, M.L.; Paíno, C.L.; Trillo, M.Á.; Úbeda, A. Electric Stimulation at 448 kHz Promotes Proliferation of Human Mesenchymal Stem Cells. Cell. Physiol. Biochem. 2014, 34, 1741–1755. [Google Scholar] [CrossRef]
- Sutermaster, B.A.; Darling, E.M. Considerations for high-yield, high-throughput cell enrichment: Fluorescence versus magnetic sorting. Sci. Rep. 2019, 9, 227. [Google Scholar] [CrossRef] [Green Version]
- Gothard, D.; Greenhough, J.; Ralph, E.; Oreffo, R.O. Prospective isolation of human bone marrow stromal cell subsets: A comparative study between Stro-1-, CD146- and CD105-enriched populations. J. Tissue Eng. 2014, 5, 2041731414551763. [Google Scholar] [CrossRef] [Green Version]
- Huynh, N.P.; Zhang, B.; Guilak, F. High-depth transcriptomic profiling reveals the temporal gene signature of human mesenchymal stem cells during chondrogenesis. FASEB J. 2019, 33, 358–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arufe, M.C.; De la Fuente, A.; Fuentes, I.; de Toro, F.J.; Blanco, F.J. Chondrogenic potential of subpopulations of cells expressing mesenchymal stem cell markers derived from human synovial membranes. J. Cell Biochem. 2010, 111, 834–845. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Li, J.; Wang, Y.; Pan, J.; Li, S.; Zhu, L.; Guo, C.; Yan, Z. CD105 promotes chondrogenesis of synovium-derived mesenchymal stem cells through Smad2 signaling. Biochem. Biophys. Res. Commun. 2016, 474, 338–344. [Google Scholar] [CrossRef]
- Lv, X.-J.; Zhou, G.-D.; Liu, Y.; Liu, X.; Chen, J.-N.; Luo, X.-S.; Cao, Y.-L. In vitro proliferation and differentiation of adipose-derived stem cells isolated using anti-CD105 magnetic beads. Int. J. Mol. Med. 2012, 30, 826–834. [Google Scholar] [CrossRef] [Green Version]
- Rada, T.; Reis, R.L.; Gomes, M.E. Distinct Stem Cells Subpopulations Isolated from Human Adipose Tissue Exhibit Different Chondrogenic and Osteogenic Differentiation Potential. Stem Cell Rev. Rep. 2011, 7, 64–76. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, G.; Giuffrida, R.; Furno, D.L.; Parrinello, N.L.; Forte, S.; Gulino, R.; Colarossi, C.; Schinocca, L.R.; Giuffrida, R.; Cardile, V.; et al. Potential Effect of CD271 on Human Mesenchymal Stromal Cell Proliferation and Differentiation. Int. J. Mol. Sci. 2015, 16, 15609–15624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petters, O.; Schmidt, C.; Thuemmler, C.; Peinemann, F.; Zscharnack, M.; Somerson, J.S.; Schulz, R.M. Point-of-care treatment of focal cartilage defects with selected chondrogenic mesenchymal stromal cells—An in vitro proof-of-concept study. J. Tissue Eng. Regen. Med. 2018, 12, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Mifune, Y.; Matsumoto, T.; Murasawa, S.; Kawamoto, A.; Kuroda, R.; Shoji, T.; Kuroda, T.; Fukui, T.; Kawakami, Y.; Kurosaka, M.; et al. Therapeutic superiority for cartilage repair by CD271-positive marrow stromal cell transplantation. Cell Transpl. 2013, 22, 1201–1211. [Google Scholar] [CrossRef]
- Kohli, N.; Al-Delfi, I.R.T.; Snow, M.; Sakamoto, T.; Miyazaki, T.; Nakajima, H.; Uchida, K.; Johnson, W.E.B. CD271-selected mesenchymal stem cells from adipose tissue enhance cartilage repair and are less angiogenic than plastic adherent mesenchymal stem cells. Sci. Rep. 2019, 9, 3194. [Google Scholar] [CrossRef] [Green Version]
- Kuçi, S.; Kuçi, Z.; Schäfer, R.; Spohn, G.; Winter, S.; Schwab, M.; Salzmann-Manrique, E.; Klingebiel, T.; Bader, P. Molecular signature of human bone marrow-derived mesenchymal stromal cell subsets. Sci. Rep. 2019, 9, 1774. [Google Scholar] [CrossRef] [Green Version]
- Halfon, S.; Abramov, N.; Grinblat, B.; Ginis, I. Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev. 2011, 20, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Park, Y.S.; Kim, H.S.; Kim, H.Y.; Jin, Y.M.; Jung, S.-C.; Ryu, K.-H.; Jo, I. Characterization of long-term in vitro culture-related alterations of human tonsil-derived mesenchymal stem cells: Role for CCN1 in replicative senescence-associated increase in osteogenic differentiation. J. Anat. 2014, 225, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Hagmann, S.; Frank, S.; Gotterbarm, T.; Dreher, T.; Eckstein, V.; Moradi, B. Fluorescence activated enrichment of CD146+ cells during expansion of human bone-marrow derived mesenchymal stromal cells augments proliferation and GAG/DNA content in chondrogenic media. BMC Musculoskelet. Disord. 2014, 15, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, X.; Zuo, W.; Wu, Z.; Chen, J.; Wu, N.; Ma, P.; Xia, Z.; Jiang, C.; Ye, Z.; Liu, S.; et al. CD146 as a new marker for an increased chondroprogenitor cell sub-population in the later stages of osteoarthritis. J. Orthop. Res. 2015, 33, 84–91. [Google Scholar] [CrossRef]
- Scioli, M.G.; Storti, G.; Bielli, A.; Sanchez, M.; Scimeca, M.; Gimble, J.M.; Cervelli, V.; Orlandi, A. CD146 expression regulates osteochondrogenic differentiation of human adipose-derived stem cells. J. Cell. Physiol. 2022, 237, 589–602. [Google Scholar] [CrossRef]
- Wu, C.-C.; Liu, F.-L.; Sytwu, H.-K.; Tsai, C.-Y.; Chang, D.-M. CD146+ mesenchymal stem cells display greater therapeutic potential than CD146– cells for treating collagen-induced arthritis in mice. Stem Cell Res. Ther. 2016, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Al Bahrawy, M. Comparison of the Migration Potential through Microperforated Membranes of CD146+ GMSC Population versus Heterogeneous GMSC Population. Stem Cells Int. 2021, 2021, 5583421. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, W.; Zha, K.; Jing, X.; Wang, M.; Zhang, Y.; Hao, C.; Gao, S.; Chen, M.; Yuan, Z.; et al. Enrichment of CD146(+) Adipose-Derived Stem Cells in Combination with Articular Cartilage Extracellular Matrix Scaffold Promotes Cartilage Regeneration. Theranostics 2019, 9, 5105–5121. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Liu, G.; Banie, L.; Wang, G.; Ning, H.; Lue, T.F.; Lin, C.-S. Tissue distribution of mesenchymal stem cell marker Stro-1. Stem Cells Dev. 2011, 20, 1747–1752. [Google Scholar] [CrossRef]
- Fitter, S.; Gronthos, S.; Ooi, S.S.; Zannettino, A.C.W. The Mesenchymal Precursor Cell Marker Antibody STRO-1 Binds to Cell Surface Heat Shock Cognate 70. Stem Cells 2017, 35, 940–951. [Google Scholar] [CrossRef] [Green Version]
- Bensidhoum, M.; Chapel, A.; Francois, S.; Demarquay, C.; Mazurier, C.; Fouillard, L.; Bouchet, S.; Bertho, J.M.; Gourmelon, P.; Aigueperse, J.; et al. Homing of in vitro expanded Stro-1- or Stro-1+ human mesenchymal stem cells into the NOD/SCID mouse and their role in supporting human CD34 cell engraftment. Blood 2004, 103, 3313–3319. [Google Scholar] [CrossRef]
- Yu, K.; Yang, S.; Jung, J.; Kim, H.; Ko, K.; Han, D.W.; Park, S.; Choi, S.W.; Kang, S.; Schöler, H.; et al. CD49f Enhances Multipotency and Maintains Stemness Through the Direct Regulation of OCT4 and SOX2. Stem Cells 2012, 30, 876–887. [Google Scholar] [CrossRef]
- Zha, K.; Li, X.; Tian, G.; Yang, Z.; Sun, Z.; Yang, Y.; Wei, F.; Huang, B.; Jiang, S.; Li, H.; et al. Evaluation of CD49f as a novel surface marker to identify functional adipose-derived mesenchymal stem cell subset. Cell Prolif. 2021, 54, e13017. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Dong, P.; Fu, X.; Li, Q.; Ma, S.; Wu, D.; Kang, N.; Liu, X.; Yan, L.; Xiao, R. CD49f Acts as an Inflammation Sensor to Regulate Differentiation, Adhesion, and Migration of Human Mesenchymal Stem Cells. Stem Cells 2015, 33, 2798–2810. [Google Scholar] [CrossRef]
- Campbell, D.D.; Pei, M. Surface Markers for Chondrogenic Determination: A Highlight of Synovium-Derived Stem Cells. Cells 2012, 1, 1107–1120. [Google Scholar] [CrossRef] [Green Version]
- Rosu-Myles, M.; McCully, J.; Fair, J.; Mehic, J.; Menendez, P.; Rodriguez, R.; Westwood, C. The globoseries glycosphingolipid SSEA-4 is a marker of bone marrow-derived clonal multipotent stromal cells in vitro and in vivo. Stem Cells Dev. 2013, 22, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Pei, M.; He, F.; Kish, V.L. Expansion on extracellular matrix deposited by human bone marrow stromal cells facilitates stem cell proliferation and tissue-specific lineage potential. Tissue Eng. Part. A. 2011, 17, 3067–3076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, A.; Sathiyanathan, P.; Yin, L.; Liu, T.M.; Lam, A.; Ravikumar, M.; Smith, R.A.A.; Loh, H.P.; Zhang, Y.; Ling, L.; et al. Strategies to enhance immunomodulatory properties and reduce heterogeneity in mesenchymal stromal cells during ex vivo expansion. Cytotherapy 2022, 24, 456–472. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, J.; Liao, J.; Zhang, F.; Zhou, G. Donor genetic backgrounds contribute to the functional heterogeneity of stem cells and clinical outcomes. Stem Cells Transl. Med. 2020, 9, 1495–1499. [Google Scholar] [CrossRef]
- Zheng, G.; Xie, Z.-Y.; Wang, P.; Wu, Y.-F.; Shen, H.-Y. Recent advances of single-cell RNA sequencing technology in mesenchymal stem cell research. World J. Stem Cells 2020, 12, 438. [Google Scholar] [CrossRef]
- Dicks, A.; Wu, C.-L.; Steward, N.; Adkar, S.S.; Gersbach, C.A.; Guilak, F. Prospective isolation of chondroprogenitors from human iPSCs based on cell surface markers identified using a CRISPR-Cas9-generated reporter. Stem Cell Res. Ther. 2020, 11, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Vertes, A. Single-cell mass spectrometry approaches to explore cellular heterogeneity. Angew. Chem. Int. Ed. 2018, 57, 4466–4477. [Google Scholar] [CrossRef]
- Wu, J.; Tzanakakis, E.S. Deconstructing stem cell population heterogeneity: Single-cell analysis and modeling approaches. Biotechnol. Adv. 2013, 31, 1047–1062. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.C.; Shi, H.; Poon, Z.; Nyan, L.M.; Kaushik, T.; Shivashankar, G.V.; Chan, J.K.Y.; Lim, C.T.; Han, J.; Van Vliet, K.J. Multivariate biophysical markers predictive of mesenchymal stromal cell multipotency. Proc. Natl. Acad. Sci. USA 2014, 111, E4409–E4418. [Google Scholar] [CrossRef] [Green Version]
- Poon, Z.; Lee, W.C.; Guan, G.; Nyan, L.M.; Lim, C.T.; Han, J.; Van Vliet, K.J. Bone marrow regeneration promoted by biophysically sorted osteoprogenitors from mesenchymal stromal cells. Stem Cells Transl. Med. 2015, 4, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Kim, D.; Tse, H.T.; Tseng, P.; Peng, L.; Dhar, M.; Karumbayaram, S.; Di Carlo, D. High-throughput physical phenotyping of cell differentiation. Microsyst. Nanoeng. 2017, 3, 17013. [Google Scholar] [CrossRef] [Green Version]
- Heo, S.J.; Driscoll, T.P.; Thorpe, S.D.; Nerurkar, N.L.; Baker, B.M.; Yang, M.T.; Chen, C.S.; Lee, D.A.; Mauck, R.L. Differentiation alters stem cell nuclear architecture, mechanics, and mechano-sensitivity. eLife 2016, 5, e18207. [Google Scholar] [CrossRef]
- Sarem, M.; Otto, O.; Tanaka, S.; Shastri, V.P. Cell number in mesenchymal stem cell aggregates dictates cell stiffness and chondrogenesis. Stem Cell Res. Ther. 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dempsey, M.E.; Chickering, G.R.; González-Cruz, R.D.; Fonseca, V.C.; Darling, E.M. Discovery of surface biomarkers for cell mechanophenotype via an intracellular protein-based enrichment strategy. Cell. Mol. Life Sci. 2022, 79, 320. [Google Scholar] [CrossRef]
- Zeming, K.K.; Vernekar, R.; Chua, M.T.; Quek, K.Y.; Sutton, G.; Krüger, T.; Kuan, W.S.; Han, J. Label-Free Biophysical Markers from Whole Blood Microfluidic Immune Profiling Reveal Severe Immune Response Signatures. Small 2021, 17, e2006123. [Google Scholar] [CrossRef]
- Yin, L.; Wu, Y.; Yang, Z.; Tee, C.A.; Denslin, V.; Lai, Z.; Lim, C.T.; Lee, E.H.; Han, J. Microfluidic label-free selection of mesenchymal stem cell subpopulation during culture expansion extends the chondrogenic potential in vitro. Lab. Chip. 2018, 18, 878–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, L.; Yang, Z.; Wu, Y.; Denslin, V.; Yu, C.C.; Tee, C.A.; Lim, C.T.; Han, J.; Lee, E.H. Label-free separation of mesenchymal stem cell subpopulations with distinct differentiation potencies and paracrine effects. Biomaterials 2020, 240, 119881. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; McBeath, R.; Chen, C.S. Stem Cell Shape Regulates a Chondrogenic Versus Myogenic Fate Through Rac1 and N-Cadherin. Stem Cells 2010, 28, 564–572. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Rowe, R.G.; Botvinick, E.L.; Kurup, A.; Putnam, A.J.; Seiki, M.; Weaver, V.M.; Keller, E.T.; Goldstein, S.; Dai, J.; et al. MT1-MMP-Dependent Control of Skeletal Stem Cell Commitment via a β1-Integrin/YAP/TAZ Signaling Axis. Dev. Cell 2013, 25, 402–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBride, S.H.; Knothe Tate, M.L. Modulation of stem cell shape and fate A: The role of density and seeding protocol on nucleus shape and gene expression. Tissue Eng. Part A 2008, 14, 1561–1572. [Google Scholar] [CrossRef]
- Zion Market Research. Insights on Global Cartilage Repair/Regeneration Market Size & Share To Hit USD 1962.53 Million by 2028, Exhibit a CAGR of 14.9%; Zion Market Research: Pune, India, 2022. [Google Scholar]
- Negoro, T.; Takagaki, Y.; Okura, H.; Matsuyama, A. Trends in clinical trials for articular cartilage repair by cell therapy. NPJ Regen. Med. 2018, 3, 17. [Google Scholar] [CrossRef] [Green Version]

| Source of Heterogeneity | Study Details | Results | Reference |
|---|---|---|---|
| Donor | BM-MSCs isolated from 53 donors (25 female, 28 male; 13 to 80 years old). | Highly clonogenic BM-MSCs were more frequent in preparations from younger female donors. | [50] |
| BM-MSCs isolated from 17 donors (25 to 81 years old). | BM-MSCs from young donors showed improved glycosaminoglycan deposition and increased expression of the chondrogenic markers SOX9, COL2A1, and ACAN. | [49] | |
| BM-MSCs isolated from donors with primary osteoarthritis, osteoporosis, and healthy donors. | BM-MSCs from patients produced chondrogenic pellets of reduced diameter. | [51] | |
| BM-MSCs isolated from donors with advanced osteoarthritis and healthy donors. | BM-MSCs from patients had a reduced proliferative capacity and a significant reduction in in vitro chondrogenic activity. | [52] | |
| Tissue | AD-MSCs and BM-MSCs isolated from the same donor. | Collagen II and proteoglycans were synthesized only in the BM-MSCs in vitro. | [54] |
| AD-MSCs, BM-MSCs, and MSCs from periosteum isolated from the same donor. | Bone marrow and periosteum yielded more homogenous MSCs than fat, improving the correction of physeal arrest in a rabbit model. | [55] | |
| BM-MSCs and AD-MSCs seeded onto two different scaffolds: Chondro-Gide or Alpha Chondro Shield. | Chondro-Gide seeded with BM-MSCs had the highest MSC proliferation and deposition of ECM tissue. | [56] | |
| BM-MSCs and AD-MSCs in a platelet-rich plasma scaffold in an osteochondral defect rabbit model. | BM-MSCs demonstrated improved morphological, histological, and immunohistochemical characteristics, higher cartilage-specific gene and protein expression, as well as subchondral bone regeneration. | [57] | |
| BM-MSCs, AD-MSCs, and cartilage-derived MSCs from adult Sprague Dawley rats. | AD-MSCs have the highest proliferation potential according to growth curve, cell cycle, and telomerase activity analyses. | [58] | |
| BM-MSCs, AD-MSCs, and UCB-MSCs. | UCB-MSCs could be cultured the longest and showed the highest proliferation capacity. | [27] | |
| Equine-derived BM-MSCs and UCB-MSCs. | BM-MSCs synthesized ECM of higher quality with a more homogenous distribution of type IIB collagen. | [59] | |
| SM-MSCs, AD-MSCs, and BM-MSCs isolated from the same donor. | SM-MSCs had the greatest potential for both proliferation and chondrogenesis. | [28] | |
| Tissue location | BM-MSCs isolated from the iliac crest, vertebral body, and femoral head. | BM-MSCs from the iliac crest and vertebral body demonstrated higher chondrogenic potential. | [61] |
| BM-MSCs isolated from femur trabeculae through rasping and from the main marrow compartment. | BM-MSCs from femur trabeculae displayed increased chondrogenic potential. | [62] | |
| AD-MSCs from superficial subcutaneous, deep subcutaneous, omentum, mesentery, and retroperitoneum. | AD-MSCs from subcutaneous tissue show increased proliferative ability and a higher level of CD146 expression. | [63] | |
| Donor-matched AD-MSCs from knee infrapatellar and subcutaneous adipose tissue of osteoarthritic donors. | AD-MSCs from the infrapatellar fat pad demonstrated increased glycosaminoglycan production and upregulation of the chondrogenic genes ACAN and COL2A1. | [64] | |
| Subpopulation | Single-cell RNA sequencing of human primary Wharton’s jelly-derived MSCs from three donors. | Differentially expressed gene analysis found several distinct subpopulations of MSCs that differ in proliferation, development, and inflammation response. | [66] |
| Single-cell RNA sequencing of BM-MSCs (three donors), AD-MSCs (three donors), UCB-MSCs (two donors), and dermis-derived MSCs (three donors). | MSC subpopulations were substantially heterogeneous in immune regulation, antigen processing/presentation, and senescence. | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, D.; Yang, Y.; Lee, E.H.; Hui, J.H.P.; Yang, Z. Managing the Heterogeneity of Mesenchymal Stem Cells for Cartilage Regenerative Therapy: A Review. Bioengineering 2023, 10, 355. https://doi.org/10.3390/bioengineering10030355
Goh D, Yang Y, Lee EH, Hui JHP, Yang Z. Managing the Heterogeneity of Mesenchymal Stem Cells for Cartilage Regenerative Therapy: A Review. Bioengineering. 2023; 10(3):355. https://doi.org/10.3390/bioengineering10030355
Chicago/Turabian StyleGoh, Doreen, Yanmeng Yang, Eng Hin Lee, James Hoi Po Hui, and Zheng Yang. 2023. "Managing the Heterogeneity of Mesenchymal Stem Cells for Cartilage Regenerative Therapy: A Review" Bioengineering 10, no. 3: 355. https://doi.org/10.3390/bioengineering10030355