Mixture Effects of Per- and Polyfluoroalkyl Substances on Embryonic and Larval Sheepshead Minnows (Cyprinodon variegatus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Maintenance of Sheepshead Minnow Culture
2.3. Exposure Regime
2.4. Oxidative Stress Assays
2.5. Gene Expression Analysis
2.6. Water Chemistry Analysis
2.7. Statistical Analyses
3. Results
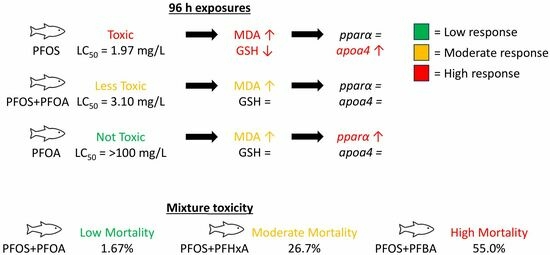
3.1. Acute Toxicity of PFOS and/or PFOA on Embryonic and Larval Sheepshead Minnows
3.2. Mixture Toxicity of PFOS with PFOA, PFHxA, or PFBA
3.3. Effects of PFAS on Gene Expression
3.4. Antagonistic Effects of PFOA on PFOS-Induced Mortality
3.5. Effects of PFOS and PFOA on Oxidative Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prevedouros, K.; Cousins, I.T.; Buck, R.C.; Korzeniowski, S.H. Sources, Fate and Transport of Perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Houde, M.; De Silva, A.O.; Muir, D.C.; Letcher, R.J. Monitoring of perfluorinated compounds in aquatic biota: An updated review: PFCs in aquatic biota. Environ. Sci. Technol. 2011, 45, 7962–7973. [Google Scholar] [CrossRef] [PubMed]
- Schaider, L.A.; Balan, S.A.; Blum, A.; Andrews, D.Q.; Strynar, M.J.; Dickinson, M.E.; Lunderberg, D.M.; Lang, J.R.; Peaslee, G.F. Fluorinated Compounds in U.S. Fast Food Packaging. Environ. Sci. Technol. Lett. 2017, 4, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Brunn, H.; Arnold, G.; Körner, W.; Rippen, G.; Steinhäuser, K.G.; Valentin, I. PFAS: Forever chemicals—Persistent, bioaccumulative and mobile. Reviewing the status and the need for their phase out and remediation of contaminated sites. Environ. Sci. Eur. 2023, 35, 20. [Google Scholar] [CrossRef]
- United Nations Environment Programme. The New POPs under the Stockholm Convention. Secretariat of the Stockholm Convention: Geneva, Switzerland, 2019. Available online: http://chm.pops.int/TheConvention/ThePOPs/TheNewPOPs (accessed on 9 January 2024).
- Lau, C.; Anitole, K.; Hodes, C.; Lai, D.; Pfahles-Hutchens, A.; Seed, J. Perfluoroalkyl Acids: A Review of Monitoring and Toxicological Findings. Toxicol. Sci. 2007, 99, 366–394. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Choi, K.; Park, K.; Seong, C.; Yu, S.D.; Kim, P. Adverse effects of perfluoroalkyl acids on fish and other aquatic organisms: A review. Sci. Total Environ. 2020, 707, 135334. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Shi, G.; Liu, C.; Hao, Q.; Wu, L. Occurrence and Risk Assessment of Perfluorooctanoate (PFOA) and Perfluorooctane Sulfonate (PFOS) in Surface Water, Groundwater and Sediments of the Jin River Basin, Southeastern China. Bull. Environ. Contam. Toxicol. 2022, 108, 1026–1032. [Google Scholar] [CrossRef]
- Takdastan, A.; Babaei, A.A.; Jorfi, S.; Ahmadi, M.; Birgani, Y.T.; Jamshidi, B. Perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) in water and edible fish species of Karun River, Ahvaz, Iran: Spatial distribution, human health, and ecological risk assessment. Int. J. Environ. Health Res. 2023, 1–12. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z. Revisiting the “forever chemicals”, PFOA and PFOS exposure in drinking water. NPJ Clean Water 2023, 6, 57. [Google Scholar] [CrossRef]
- Jones, D.K.; Quinlin, K.A.; Wigren, M.A.; Choi, Y.J.; Sepúlveda, M.S.; Lee, L.S.; Haskins, D.L.; Lotufo, G.R.; Kennedy, A.; May, L.; et al. Acute toxicity of eight aqueous film-forming foams to 14 aquatic species. Environ. Sci. Technol. 2022, 56, 6078–6090. [Google Scholar] [CrossRef]
- Seals, S. Phase I Final Rule and Technical Development Document of Uniform National Discharge Standards (UNDS) Stern Tube Seals and Underwater Bearing Lubrication: Nature of Discharge; 1999. Available online: https://www.epa.gov/vessels-marinas-and-ports/uniform-national-discharge-standards-unds-phase-i-final-rule (accessed on 9 January 2024).
- Cui, D.; Li, X.; Quinete, N. Occurrence, fate, sources and toxicity of PFAS: What we know so far in Florida and major gaps. TrAC Trends Anal. Chem. 2020, 130, 115976. [Google Scholar] [CrossRef]
- Anderson, R.H.; Long, G.C.; Porter, R.C.; Anderson, J.K. Occurrence of select perfluoroalkyl substances at US Air Force aqueous film-forming foam release sites other than fire training areas: Field validation of critical fate and transport properties. In Perfluoroalkyl Substances in the Environment; CRC Press: Boca Raton, FL, USA, 2018; pp. 353–372. [Google Scholar]
- United States Environmental Protection Agency (U.S. EPA). Draft Aquatic Life Ambient Water Quality Criteria for Per-Fluorooctane Sulfonate (PFOS); EPA 842-S-22-002; Office of Water, Office of Science and Technology, Health and Ecological Criteria Division: Washington, DC, USA, 2022. [Google Scholar]
- Haukås, M.; Berger, U.; Hop, H.; Gulliksen, B.; Gabrielsen, G.W. Bioaccumulation of per- and polyfluorinated alkyl substances (PFAS) in selected species from the Barents Sea food web. Environ. Pollut. 2007, 148, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Goeritz, I.; Falk, S.; Stahl, T.; Schäfers, C.; Schlechtriem, C. Biomagnification and tissue distribution of perfluoroalkyl substances (PFASs) in market-size rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 2013, 32, 2078–2088. [Google Scholar] [CrossRef] [PubMed]
- Miranda, D.A.; Zachritz, A.M.; Whitehead, H.D.; Cressman, S.R.; Peaslee, G.F.; Lamberti, G.A. Occurrence and biomagnification of perfluoroalkyl substances (PFAS) in Lake Michigan fishes. Sci. Total Environ. 2023, 895, 164903. [Google Scholar] [CrossRef] [PubMed]
- Ankley, G.T.; Cureton, P.; Hoke, R.A.; Houde, M.; Kumar, A.; Kurias, J.; Lanno, R.; McCarthy, C.; Newsted, J.; Salice, C.J. Assessing the ecological risks of per-and polyfluoroalkyl substances: Current state-of-the science and a proposed path forward. Environ. Toxicol. Chem. 2021, 40, 564–605. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, C.; Gibbes, B.; Wang, T.; Lockington, D. Coupling effects of tide and salting-out on perfluorooctane sulfonate (PFOS) transport and adsorption in a coastal aquifer. Adv. Water Resour. 2022, 166, 104240. [Google Scholar] [CrossRef]
- Navarro, D.A.; Oliver, D.P.; Simpson, S.L.; Kookana, R.S. Organic carbon and salinity affect desorption of PFAS from estuarine sediments. J. Soils Sediments 2022, 22, 1302–1314. [Google Scholar] [CrossRef]
- Yin, C.; Pan, C.-G.; Xiao, S.-K.; Wu, Q.; Tan, H.-M.; Yu, K. Insights into the effects of salinity on the sorption and desorption of legacy and emerging per-and polyfluoroalkyl substances (PFASs) on marine sediments. Environ. Pollut. 2022, 300, 118957. [Google Scholar] [CrossRef]
- Morrison, A.L.; Strezov, V.; Niven, R.K.; Taylor, M.P.; Wilson, S.P.; Wang, J.; Burns, D.J.; Murphy, P.J.C. Impact of Salinity and Temperature on Removal of PFAS Species from Water by Aeration in the Absence of Additional Surfactants: A Novel Application of Green Chemistry Using Adsorptive Bubble Fractionation. Ind. Eng. Chem. Res. 2023, 62, 5635–5645. [Google Scholar] [CrossRef]
- Burcham, L.E.; Allmon, E.B.; Scherer, M.N.; Bushong, A.G.; Hamilton, M.T.; Macheri, S.; Coogan, G.S.; Choi, Y.; Lee, L.; Sepúlveda, M.S. Does Salinity Mediate the Toxicity of Perfluorooctanesulfonic Acid (Pfos) in an Estuarine Fish? Available online: https://ssrn.com/abstract=4409246 (accessed on 9 January 2024).
- Abbott, B.D. Mechanisms of perfluoroalkyl acid (PFAA) toxicity: Involvement of peroxisome proliferator activator receptor alpha (PPARα) molecular signals. Reprod. Toxicol. 2009, 27, 409. [Google Scholar] [CrossRef]
- Rosen, M.B.; Das, K.P.; Rooney, J.; Abbott, B.; Lau, C.; Corton, J.C. PPARα-independent transcriptional targets of perfluoroalkyl acids revealed by transcript profiling. Toxicology 2017, 387, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.J.; Takacs, M.L.; Schmid, J.E.; Lau, C.; Abbott, B.D. Activation of mouse and human peroxisome prolifer-ator− activated receptor alpha by perfluoroalkyl acids of different functional groups and chain lengths. Toxicol. Sci. 2008, 106, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhou, B. The Role of Nrf2 and MAPK Pathways in PFOS-Induced Oxidative Stress in Zebrafish Embryos. Toxicol. Sci. 2010, 115, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Sant, K.E.; Sinno, P.P.; Jacobs, H.M.; Timme-Laragy, A.R. Nrf2a modulates the embryonic antioxidant response to perfluorooctanesulfonic acid (PFOS) in the zebrafish, Danio rerio. Aquat. Toxicol. 2018, 198, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Soto-Moreno, E.J.; Prakash, A.; Balboula, A.Z.; Qiao, H. Adverse PFAS effects on mouse oocyte in vitro maturation are associated with carbon-chain length and inclusion of a sulfonate group. Cell Prolif. 2023, 56, e13353. [Google Scholar] [CrossRef] [PubMed]
- 3M. The Science of Organic Fluorochemistry; Internal document; OPPT-2002-0043-0006; 3M: St. Paul, MN, USA, 1999. [Google Scholar]
- Ding, G.; Zhang, J.; Chen, Y.; Wang, L.; Wang, M.; Xiong, D.; Sun, Y. Combined Effects of PFOS and PFOA on Zebrafish (Danio rerio) Embryos. Arch. Environ. Contam. Toxicol. 2013, 64, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.W.; Chislock, M.F.; Gannon, M.E.; Bauer, S.J.; Tornabene, B.J.; Hoverman, J.T.; Sepúlveda, M.S. Acute and chronic effects of perfluoroalkyl substance mixtures on larval American bullfrogs (Rana catesbeiana). Chemosphere 2019, 236, 124350. [Google Scholar] [CrossRef]
- Foguth, R.; Hoskins, T.; Clark, G.; Nelson, M.; Flynn, R.; de Perre, C.; Hoverman, J.; Lee, L.; Sepúlveda, M.; Cannon, J. Single and mixture per- and polyfluoroalkyl substances accumulate in developing Northern leopard frog brains and produce complex neurotransmission alterations. Neurotoxicol. Teratol. 2020, 81, 106907. [Google Scholar] [CrossRef]
- Menger, F.; Pohl, J.; Ahrens, L.; Carlsson, G.; Örn, S. Behavioural effects and bioconcentration of per- and polyfluoroalkyl substances (PFASs) in zebrafish (Danio rerio) embryos. Chemosphere 2020, 245, 125573. [Google Scholar] [CrossRef]
- Grønnestad, R.; Johanson, S.M.; Müller, M.H.; Schlenk, D.; Tanabe, P.; Krøkje, Å.; Jaspers, V.L.; Jenssen, B.M.; Ræder, E.M.; Lyche, J.L.; et al. Effects of an environmentally relevant PFAS mixture on dopamine and steroid hormone levels in exposed mice. Toxicol. Appl. Pharmacol. 2021, 428, 115670. [Google Scholar] [CrossRef]
- Dennis, N.M.; Karnjanapiboonwong, A.; Subbiah, S.; Rewerts, J.N.; Field, J.A.; McCarthy, C.; Salice, C.J.; Anderson, T.A. Chronic Reproductive Toxicity of Perfluorooctane Sulfonic Acid and a Simple Mixture of Perfluorooctane Sulfonic Acid and Perfluorohexane Sulfonic Acid to Northern Bobwhite Quail (Colinus virginianus). Environ. Toxicol. Chem. 2020, 39, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Dennis, N.M.; Subbiah, S.; Karnjanapiboonwong, A.; Dennis, M.L.; McCarthy, C.; Salice, C.J.; Anderson, T.A. Species- and Tissue-Specific Avian Chronic Toxicity Values for Perfluorooctane Sulfonate (PFOS) and a Binary Mixture of PFOS and Perfluorohexane Sulfonate. Environ. Toxicol. Chem. 2021, 40, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Dennis, N.M.; Hossain, F.; Subbiah, S.; Karnjanapiboonwong, A.; Dennis, M.L.; McCarthy, C.; Jackson, W.A.; Crago, J.P.; Salice, C.J.; Anderson, T.A. Species- and Tissue-Specific Chronic Toxicity Values for Northern Bobwhite Quail (Colinus virginianus) Exposed to Perfluorohexane Sulfonic Acid and a Binary Mixture of Perfluorooctane Sulfonic Acid and Perfluorohexane Sulfonic Acid. Environ. Toxicol. Chem. 2022, 41, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Method E 1241-98; Standard Guide for Conducting Early Life-Stage Toxicity Tests with Fishes. American Society for Testing and Materials: West Conshohocken, PA, USA, 2004.
- DeLorenzo, M.E.; Evans, B.N.; Chung, K.W.; Key, P.B.; Fulton, M.H. Effects of salinity on oil dispersant toxicity in the eastern mud snail, Ilyanassa obsoleta. Environ. Sci. Pollut. Res. 2017, 24, 21476–21483. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.W.; Beers, D.A.; Pisarski, E.C.; Aquilina-Beck, A.; Pennington, P.L.; DeLorenzo, M.E. Effects of Photoenhanced Thin Oil Sheens on Survival and Growth of Newly Hatched Fishes: Sheepshead Minnow (Cyprinodon variegatus), spotted seatrout (Cynoscion nebulosus), and Red Drum (Sciaenops ocellatus). Aquac. Fish. Stud. 2022, 4, 1–11. [Google Scholar]
- Ringwood, A.H.; Hoguet, J.; Keppler, C.J.; Gielazyn, M.L.; Ward, B.P.; Rourk, A.R. Cellular Biomarkers (Lysosomal Destabilization, Glutathione & Lipid Peroxidation) in Three Common Estuarine Species: A Methods Handbook; Marine Resources Research Institute, South Carolina Department of Natural Resources: Charleston, SC, USA, 2003. [Google Scholar]
- Hoguet, J.; Key, P.B. Activities of biomarkers in multiple life stages of the model crustacean, Palaemonetes pugio. J. Exp. Mar. Biol. Ecol. 2007, 353, 235–244. [Google Scholar] [CrossRef]
- Aquilina-Beck, A.A.; Reiner, J.L.; Chung, K.W.; DeLise, M.J.; Key, P.B.; DeLorenzo, M.E. Uptake and Biological Effects of Perfluorooctane Sulfonate Exposure in the Adult Eastern Oyster Crassostrea virginica. Arch. Environ. Contam. Toxicol. 2020, 79, 333–342. [Google Scholar] [CrossRef]
- Magnuson, J.T.; Giroux, M.; Cryder, Z.; Gan, J.; Schlenk, D. The use of non-targeted metabolomics to assess the toxicity of bifenthrin to juvenile Chinook salmon (Oncorhynchus tshawytscha). Aquat. Toxicol. 2020, 224, 105518. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−#x394;ΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Nagasawa, M.; Akasaka, Y.; Ide, T.; Hara, T.; Kobayashi, N.; Utsumi, M.; Murakami, K. Highly sensitive upregulation of apolipoprotein A-IV by peroxisome proliferator-activated receptor α (PPARα) agonist in human hepatoma cells. Biochem. Pharmacol. 2007, 74, 1738–1746. [Google Scholar] [CrossRef]
- Tanabe, P.; Schlenk, D. Role of Aryl Hydrocarbon Receptor and Oxidative Stress in the Regioselective Toxicities of Hydroxychrysenes in Embryonic Japanese Medaka (Oryzias latipes). Environ. Toxicol. Chem. 2023, 42, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Tokusumi, H.; Fujioka, N.; Tue, N.M.; Goto, A.; Suzuki, G.; Nakayama, K. Toxicity testing of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin in early-life stage of Japanese medaka: Optimization of conditions for assessing relative potencies of dioxin-like compounds. Jpn. J. Environ. Toxicol. 2021, 24, 1–11. [Google Scholar]
- Ji, C.; Tanabe, P.; Shi, Q.; Qian, L.; McGruer, V.; Magnuson, J.T.; Wang, X.; Gan, J.; Gadepalli, R.S.; Rimoldi, J.; et al. Stage Dependent Enantioselective Metabolism of Bifenthrin in Embryos of Zebrafish (Danio rerio) and Japanese Medaka (Oryzias latipes). Environ. Sci. Technol. 2021, 55, 9087–9096. [Google Scholar] [CrossRef] [PubMed]
- Embry, M.R.; Belanger, S.E.; Braunbeck, T.A.; Galay-Burgos, M.; Halder, M.; Hinton, D.E.; Léonard, M.A.; Lillicrap, A.; Norberg-King, T.; Whale, G. The fish embryo toxicity test as an animal alternative method in hazard and risk as-sessment and scientific research. Aquat. Toxicol. 2010, 97, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, L.; Wong, C.K.C.; Cheng, S.H. Rapid adaptation of molecular resources from zebrafish and medaka to develop an estuarine/marine model. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Pelka, K.E. The Applicability of the Fish Embryo Toxicity Test (FET) for the Testing of Chemical Substances with Particular Reference to a Possible Barrier Function of the Chorion. Ph.D. Thesis, The Faculty of Bio Sciences, Dean’s Office of the Faculty of Bio Sciences, Frankfurt, Germany, 2017. [Google Scholar]
- Ankley, G.T.; Kuehl, D.W.; Kahl, M.D.; Jensen, K.M.; Linnum, A.; Leino, R.L.; Villeneuve, D.A. Reproductive and de-velopmental toxicity and bioconcentration of perfluorooctanesulfonate in a partial life-cycle test with the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. Int. J. 2005, 24, 2316–2324. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Wu, X.; Huang, Q.; Liao, Y.; Liu, L.; Qiu, L.; Shen, H.; Dong, S. PFOS elicits transcriptional responses of the ER, AHR and PPAR pathways in Oryzias melastigma in a stage-specific manner. Aquat. Toxicol. 2012, 106–107, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Qiu, W.; Zhang, S.; Wang, J.; Yang, X.; Xu, B.; Magnuson, J.T.; Xu, E.G.; Wu, M.; Zheng, C. Poly- and Perfluoroalkyl Substances Induce Immunotoxicity via the TLR Pathway in Zebrafish: Links to Carbon Chain Length. Environ. Sci. Technol. 2023, 15, 6139–6149. [Google Scholar] [CrossRef]
- Behr, A.-C.; Plinsch, C.; Braeuning, A.; Buhrke, T. Activation of human nuclear receptors by perfluoroalkylated substances (PFAS). Toxicol. In Vitro 2020, 62, 104700. [Google Scholar] [CrossRef]
- Evans, N.; Conley, J.M.; Cardon, M.; Hartig, P.; Medlock-Kakaley, E.; Gray, L.E., Jr. In vitro activity of a panel of per-and polyfluoroalkyl substances (PFAS), fatty acids, and pharmaceuticals in peroxisome proliferator-activated receptor (PPAR) alpha, PPAR gamma, and estrogen receptor assays. Toxicol. Appl. Pharmacol. 2022, 449, 116136. [Google Scholar] [CrossRef]
- Takacs, M.L.; Abbott, B.D. Activation of mouse and human peroxisome proliferator–activated receptors (α, β/δ, γ) by perfluorooctanoic acid and perfluorooctane sulfonate. Toxicol. Sci. 2007, 95, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Bjork, J.A.; Wallace, K.B. Structure-Activity Relationships and Human Relevance for Perfluoroalkyl Acid–Induced Transcriptional Activation of Peroxisome Proliferation in Liver Cell Cultures. Toxicol. Sci. 2009, 111, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, G.; Heiger-Bernays, W.J.; Schlezinger, J.J.; Webster, T.F. Predicting the effects of per- and polyfluoroalkyl substance mixtures on peroxisome proliferator-activated receptor alpha activity in vitro. Toxicology 2022, 465, 153024. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, H.; Hirano, M.; Kim, E.-Y.; Iwata, H. In Vitro and In Silico Evaluations of Binding Affinities of Perfluoroalkyl Substances to Baikal Seal and Human Peroxisome Proliferator-Activated Receptor α. Environ. Sci. Technol. 2019, 53, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Søderstrøm, S.; Lille-Langøy, R.; Yadetie, F.; Rauch, M.; Milinski, A.; Dejaegere, A.; Stote, R.H.; Goksøyr, A.; Karlsen, O.A. Agonistic and potentiating effects of perfluoroalkyl substances (PFAS) on the Atlantic cod (Gadus morhua) peroxisome proliferator-activated receptors (Ppars). Environ. Int. 2022, 163, 107203. [Google Scholar] [CrossRef] [PubMed]
- Arukwe, A.; Mortensen, A.S. Lipid peroxidation and oxidative stress responses of salmon fed a diet containing perfluorooctane sulfonic- or perfluorooctane carboxylic acids. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 154, 288–295. [Google Scholar] [CrossRef]
- Hagenaars, A.; Vergauwen, L.; Benoot, D.; Laukens, K.; Knapen, D. Mechanistic toxicity study of perfluorooctanoic acid in zebrafish suggests mitochondrial dysfunction to play a key role in PFOA toxicity. Chemosphere 2013, 91, 844–856. [Google Scholar] [CrossRef]
- Fang, X.; Wei, Y.; Liu, Y.; Wang, J.; Dai, J. The identification of apolipoprotein genes in rare minnow (Gobiocypris rarus) and their expression following perfluorooctanoic acid exposure. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 151, 152–159. [Google Scholar] [CrossRef]
- Cui, Y.; Lv, S.; Liu, J.; Nie, S.; Chen, J.; Dong, Q.; Huang, C.; Yang, D. Chronic perfluorooctanesulfonic acid exposure disrupts lipid metabolism in zebrafish. Hum. Exp. Toxicol. 2017, 36, 207–217. [Google Scholar] [CrossRef]
- Naile, J.E.; Wiseman, S.; Bachtold, K.; Jones, P.D.; Giesy, J.P. Transcriptional effects of perfluorinated compounds in rat hepatoma cells. Chemosphere 2012, 86, 270–277. [Google Scholar] [CrossRef]
- Dale, K.; Yadetie, F.; Müller, M.B.; Pampanin, D.M.; Gilabert, A.; Zhang, X.; Tairova, Z.; Haarr, A.; Lille-Langøy, R.; Lyche, J.L.; et al. Proteomics and lipidomics analyses reveal modulation of lipid metabolism by perfluoroalkyl substances in liver of Atlantic cod (Gadus morhua). Aquat. Toxicol. 2020, 227, 105590. [Google Scholar] [CrossRef] [PubMed]
- Glaser, D.; Lamoureux, E.; Opdyke, D.; LaRoe, S.; Reidy, D.; Connolly, J. The impact of precursors on aquatic exposure assessment for PFAS: Insights from bioaccumulation modeling. Integr. Environ. Assess. Manag. 2021, 17, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhang, X.; Qiao, Y.; Griffin, N.; Zhang, H.; Wang, L.; Liu, H. Exposure to PFOA and its novel analogs disrupts lipid metabolism in zebrafish. Ecotoxicol. Environ. Saf. 2023, 259, 115020. [Google Scholar] [CrossRef] [PubMed]
- Point, A.D.; Crimmins, B.S.; Holsen, T.M.; Fernando, S.; Hopke, P.K.; Darie, C.C. Can blood proteome diversity among fish species help explain perfluoroalkyl acid trophodynamics in aquatic food webs? Sci. Total Environ. 2023, 875, 162337. [Google Scholar] [CrossRef] [PubMed]
- Haug, M.; Dunder, L.; Lind, P.M.; Lind, L.; Salihovic, S. Associations of perfluoroalkyl substances (PFAS) with lipid and lipoprotein profiles. J. Expo. Sci. Environ. Epidemiol. 2023, 33, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Jiang, X.-C.; Liu, R.; Liang, G.; Beyer, T.P.; Gao, H.; Ryan, T.P.; Li, S.D.; Eacho, P.I.; Cao, G. Liver X Receptors (LXRs) Regulate Apolipoprotein AIV-Implications of the Antiatherosclerotic Effect of LXR Agonists. Mol. Endocrinol. 2004, 18, 2000–2010. [Google Scholar] [CrossRef]
- Pinto, C.L.; Kalasekar, S.M.; McCollum, C.W.; Riu, A.; Jonsson, P.; Lopez, J.; Swindell, E.C.; Bouhlatouf, A.; Balaguer, P.; Bondesson, M.; et al. Lxr regulates lipid metabolic and visual perception pathways during zebrafish development. Mol. Cell. Endocrinol. 2016, 419, 29–43. [Google Scholar] [CrossRef]
- Reddy, J.K.; Hashimoto, T. Peroxisomal β-oxidation and peroxisome proliferator–activated receptor α: An adaptive metabolic system. Annu. Rev. Nutr. 2001, 21, 193–230. [Google Scholar] [CrossRef]
- Taibl, K.R.; Schantz, S.; Aung, M.T.; Padula, A.; Geiger, S.; Smith, S.; Park, J.S.; Milne, G.L.; Robinson, J.F.; Woodruff, T.J.; et al. Associations of per-and polyfluoroalkyl substances (PFAS) and their mixture with oxidative stress biomarkers during pregnancy. Environ. Int. 2022, 169, 107541. [Google Scholar] [CrossRef]




| Chemical | Embryonic LC50 (mg/L) | Larval LC50 (mg/L) |
|---|---|---|
| PFOS | >10 | 1.97 (1.64–2.16) |
| PFOA | >100 | >100 |
| PFOS + PFOA (1:1) | >10 | 1.99 (1.70–2.17) |
| PFOS + 100 mg/L PFOA | >10 | 3.10 (2.62–3.79) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanabe, P.; Key, P.B.; Chung, K.W.; Pisarski, E.C.; Reiner, J.L.; Rodowa, A.E.; Magnuson, J.T.; DeLorenzo, M.E. Mixture Effects of Per- and Polyfluoroalkyl Substances on Embryonic and Larval Sheepshead Minnows (Cyprinodon variegatus). Toxics 2024, 12, 91. https://doi.org/10.3390/toxics12010091
Tanabe P, Key PB, Chung KW, Pisarski EC, Reiner JL, Rodowa AE, Magnuson JT, DeLorenzo ME. Mixture Effects of Per- and Polyfluoroalkyl Substances on Embryonic and Larval Sheepshead Minnows (Cyprinodon variegatus). Toxics. 2024; 12(1):91. https://doi.org/10.3390/toxics12010091
Chicago/Turabian StyleTanabe, Philip, Peter B. Key, Katy W. Chung, Emily C. Pisarski, Jessica L. Reiner, Alix E. Rodowa, Jason T. Magnuson, and Marie E. DeLorenzo. 2024. "Mixture Effects of Per- and Polyfluoroalkyl Substances on Embryonic and Larval Sheepshead Minnows (Cyprinodon variegatus)" Toxics 12, no. 1: 91. https://doi.org/10.3390/toxics12010091