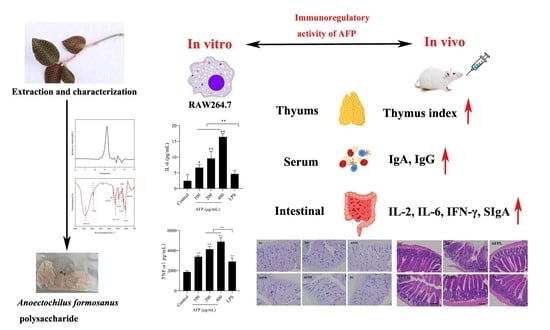
Protective Effect of Anoectochilus formosanus Polysaccharide against Cyclophosphamide-Induced Immunosuppression in BALB/c Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Polysaccharides
2.3. Homogeneity and Molecular Weight Distribution of AFP
2.4. Determination of Chemical Components
2.5. Monosaccharide Composition Analysis
2.6. Fourier Transform Infrared Spectrum
2.7. Cell Culture
2.7.1. Cell Viability
2.7.2. Influence of AFP on TNF-α and IL-6 Production of RAW 264.7
2.7.3. Western Blot Analysis
2.8. Animals’ Experiments
2.8.1. Animals and Treatment
2.8.2. Influence of AFP on Body Weight and Thymus Index
2.8.3. Preparation and Staining of Intestinal Section
2.8.4. Analysis of Serum Immunoglobulin A (IgA) and Immunoglobulin G (IgG) Secretion
2.8.5. Analysis of Small Intestinal Cytokines Level
2.9. Statistical Analysis
3. Results
3.1. Characterization and Identification of Polysaccharide from AF
3.2. Monosaccharide Composition Analysis
3.3. Fourier Transform Infrared Spectrum
3.4. In Vitro Immunostimulatory Activities on Macrophages of AFP
3.5. Activation of the NF-κB Signaling Pathway by AFP
3.6. Influence of AFP on Body Weight and Thymus Index
3.7. Effect of AFP on Intestine Tissue
3.8. Effect of AFP on the Goblet Cells and PAS-Positive Area
3.9. Effects of AFP on IgA and IgG Levels in the Serum
3.10. Effects of AFP on the Cytokine Levels in the Small Intestine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Ahlmann, M.; Hempel, G. The effect of cyclophosphamide on the immune system: Implications for clinical cancer therapy. Cancer Chemother. Pharmacol. 2016, 78, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Qu, H.; Gao, Z.; Zeng, D.; Wang, J.; Baranenko, D.; Li, Y.; Lu, W. Protective effects of Ulva pertusa polysaccharide and polysaccharideiron (III) complex on cyclophosphamide induced immunosuppression in mice. Int. J. Biol. Macromol. 2019, 133, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Duan, S.; Li, Y.; Pan, X.; Han, L. Polysaccharides in natural products that repair the damage to intestinal mucosa caused by cyclophosphamide and their mechanisms: A review. Carbohydr. Polym. 2021, 261, 117876. [Google Scholar] [CrossRef]
- Chen, X.; Nie, W.; Fan, S.; Zhang, J.; Wang, Y.; Lu, J.; Jin, L. A polysaccharide from Sargassum fusiforme protects against immunosuppression in cyclophosphamide-treated mice. Carbohydr. Polym. 2012, 90, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yan, Y.M.; Zhou, W.T.; Chen, D.; Huang, K.Y.; Yu, S.J.; Mi, J.; Lu, L.; Zeng, X.X.; Cao, Y.L. Effects of polysaccharides from bee collected pollen of Chinese wolfberry on immune response and gut microbiota composition in cyclophosphamide-treated mice. J. Funct. Foods 2020, 72, 104057. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, F.; Zhang, R.; Dong, L.; Jia, X.; Liu, L.; Yi, Y.; Zhang, M. Longan pulp polysaccharides relieve intestinal injury in vivo and in vitro by promoting tight junction expression. Carbohydr. Polym. 2020, 229, 115475. [Google Scholar] [CrossRef] [PubMed]
- Besednova, N.N.; Zaporozhets, T.S.; Kuznetsova, T.A.; Makarenkova, I.D.; Kryzhanovsky, S.P.; Fedyanina, L.N.; Ermakova, S.P. Extracts and Marine Algae Polysaccharides in Therapy and Prevention of Inflammatory Diseases of the Intestine. Mar. Drugs 2020, 18, 289. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Meng, J.; Huang, X.; Wu, G.; Zuo, S.; Nie, S. Comparative study on antidiabetic function of six legume crude polysaccharides. Int. J. Biol. Macromol. 2020, 154, 25–30. [Google Scholar] [CrossRef]
- Chen, X.; Cai, B.; Wang, J.; Sheng, Z.; Yang, H.; Wang, D.; Chen, J.; Ning, Q. Mulberry leaf-derived polysaccharide modulates the immune response and gut microbiota composition in immunosuppressed mice. J. Funct. Foods 2021, 83, 104545. [Google Scholar] [CrossRef]
- Wen, L.; Sheng, Z.; Wang, J.; Jiang, Y.; Yang, B. Structure of water-soluble polysaccharides in spore of Ganoderma lucidum and their anti-inflammatory activity. Food Chem. 2022, 373, 131374. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, X.; Zhang, L.; Zeng, F. Chain conformation and anti-tumor activities of phosphorylated (1→3)-β-d-glucan from Poria cocos. Carbohydr. Polym. 2009, 78, 581–587. [Google Scholar] [CrossRef]
- Chen, S.J.; Li, J.Y.; Zhang, J.M. Extraction of yellow pear residue polysaccharides and effects on immune function and antioxidant activity of immunosuppressed mice. Int. J. Biol. Macromol. 2019, 126, 1273–1281. [Google Scholar] [CrossRef]
- Gao, H.; Ding, L.; Liu, R.; Zheng, X.; Xia, X.; Wang, F.; Qi, J.; Tong, W.; Qiu, Y. Characterization of Anoectochilus roxburghii polysaccharide and its therapeutic effect on type 2 diabetic mice. Int. J. Biol. Macromol. 2021, 179, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, T.; Duan, S.; Li, C.; Lin, Q.; Wu, H.; Liu, A.; Hu, B.; Wu, D.; Li, S.; et al. The purification, structural characterization and antidiabetic activity of a polysaccharide from Anoectochilus roxburghii. Food Funct. 2020, 11, 3730–3740. [Google Scholar] [CrossRef]
- Wu, T.; Li, S.; Huang, Y.; He, Z.; Zheng, Y.; Stalin, A.; Shao, Q.; Lin, D. Structure and pharmacological activities of polysaccharides from Anoectochilus roxburghii (Wall.) Lindl. J. Funct. Foods 2021, 87, 104815. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, C.; Jiang, Y.; Bai, B.; He, X.; Wang, H.; Wu, J.; Zheng, C. Structural characterization and hepatoprotective effects of polysaccharides from Anoectochilus zhejiangensis. Int. J. Biol. Macromol. 2022, 198, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.C.; Shang, H.F.; Wang, L.F.; Su, B.; Hsu, C.C.; Kao, H.Y.; Cheng, K.T. Antitumor and immunostimulating effects of Anoectochilus formosanus Hayata. Phytomedicine 2006, 13, 366–370. [Google Scholar] [CrossRef]
- Ho, Y.; Chen, Y.F.; Wang, L.H.; Hsu, K.Y.; Chin, Y.T.; Yang, Y.S.H.; Wang, S.H.; Chen, Y.R.; Shih, Y.J.; Liu, L.F.; et al. Inhibitory Effect of Anoectochilus formosanus Extract on Hyperglycemia-Related PD-L1 Expression and Cancer Proliferation. Front. Pharmacol. 2018, 9, 807. [Google Scholar] [CrossRef]
- Tang, T.; Duan, X.; Ke, Y.; Zhang, L.; Shen, Y.; Hu, B.; Liu, A.; Chen, H.; Li, C.; Wu, W.; et al. Antidiabetic activities of polysaccharides from Anoectochilus roxburghii and Anoectochilus formosanus in STZ-induced diabetic mice. Int. J. Biol. Macromol. 2018, 112, 882–888. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, L.; Yan, A.; Feng, L.; Wan, Y. Fractionation, structure and conformation characterization of polysaccharides from Anoectochilus roxburghii. Carbohydr. Polym. 2020, 231, 115688. [Google Scholar] [CrossRef]
- Zhang, W.N.; Gong, L.L.; Liu, Y.; Zhou, Z.B.; Wan, C.X.; Xu, J.J.; Wu, Q.X.; Chen, L.; Lu, Y.M.; Chen, Y. Immunoenhancement effect of crude polysaccharides of Helvella leucopus on cyclophosphamide-induced immunosuppressive mice. J. Funct. Foods 2020, 69, 103942. [Google Scholar] [CrossRef]
- Liu, X.; Ren, Z.; Yu, R.; Chen, S.; Zhang, J.; Xu, Y.; Meng, Z.; Luo, Y.; Zhang, W.; Huang, Y.; et al. Structural characterization of enzymatic modification of Hericium erinaceus polysaccharide and its immune-enhancement activity. Int. J. Biol. Macromol. 2021, 166, 1396–1408. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yin, J.; Nie, S.; Wan, Y.; Xie, M. Fractionation, physicochemical property and immunological activity of polysaccharides from Cassia obtusifolia. Int. J. Biol. Macromol. 2016, 91, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Blumenkratz, N.; Asboe-Hansen, G. New Method for Quantitative Determination of Uranic Acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zeng, H.; Chen, J.; Zhai, J.; Wang, H.; Xia, W.; Xiong, Y. Reduction of the fat content of battered and breaded fish balls during deep-fat frying using fermented bamboo shoot dietary fiber. LWT 2016, 73, 425–431. [Google Scholar] [CrossRef]
- Li, G.; Chen, P.; Zhao, Y.; Zeng, Q.; Ou, S.; Zhang, Y.; Wang, P.; Chen, N.; Ou, J. Isolation, structural characterization and anti-oxidant activity of a novel polysaccharide from garlic bolt. Carbohydr. Polym. 2021, 267, 118194. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Dong, Z.; Zhu, X.; Xu, H.; Zhao, Z. Characterization and protective effect of Polygonatum sibiricum polysaccharide against cyclophosphamide-induced immunosuppression in Balb/c mice. Int. J. Biol. Macromol. 2018, 107, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, J.; Fang, Q.; Dong, N.; Nie, S. Polysaccharide from natural Cordyceps sinensis ameliorated intestinal injury and enhanced antioxidant activity in immunosuppressed mice. Food Hydrocoll. 2019, 89, 661–667. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.; Wang, Y.; Jin, W.; Guo, Y. The immunoenhancement effects of starfish Asterias rollestoni polysaccharides in macrophages and cyclophosphamide-induced immunosuppression mouse models. Food Funct. 2020, 11, 10700–10708. [Google Scholar] [CrossRef]
- Omar-Aziz, M.; Yarmand, M.S.; Khodaiyan, F.; Mousavi, M.; Gharaghani, M.; Kennedy, J.F.; Hosseini, S.S. Chemical modification of pullulan exopolysaccharide by octenyl succinic anhydride: Optimization, physicochemical, structural and functional properties. Int. J. Biol. Macromol. 2020, 164, 3485–3495. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, S.; Ying, H.; Dai, X.; Li, X.; Yu, C.; Ye, H. Chemical characterization and immunostimulatory effects of a polysaccharide from Polygoni Multiflori Radix Praeparata in cyclophosphamide-induced anemic mice. Carbohydr. Polym. 2012, 88, 1476–1482. [Google Scholar] [CrossRef]
- Xu, Y.; Cai, F.; Yu, Z.; Zhang, L.; Li, X.; Yang, Y.; Liu, G. Optimisation of pressurised water extraction of polysaccharides from blackcurrant and its antioxidant activity. Food Chem. 2016, 194, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Castro-Alves, V.C.; do Nascimento, J.R.O. Polysaccharides from raw and cooked chayote modulate macrophage function. Food Res. Int. 2016, 81, 171–179. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, Y.; Li, H. Advances in Research on Immunoregulation of Macrophages by Plant Polysaccharides. Front. Immunol. 2019, 10, 145. [Google Scholar] [CrossRef]
- Ren, D.; Zhao, Y.; Zheng, Q.; Alim, A.; Yang, X. Immunomodulatory effects of an acidic polysaccharide fraction from herbal Gynostemma pentaphyllum tea in RAW264.7 cells. Food Funct. 2019, 10, 2186–2197. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Lu, J.; Zhan, L.; Zheng, D.; Wang, Y.; Meng, J.; Li, P.; Zhao, J.; Zhang, W. An Alkali-extracted polysaccharide from Poria cocos activates RAW264.7 macrophages via NF-κB signaling pathway. Arab. J. Chem. 2023, 16, 104592. [Google Scholar] [CrossRef]
- Zhang, Q.; Cong, R.; Hu, M.; Zhu, Y.; Yang, X. Immunoenhancement of Edible Fungal Polysaccharides (Lentinan, Tremellan, and Pachymaran) on Cyclophosphamide-Induced Immunosuppression in Mouse Model. Evid.-Based Complement. Altern. Med. 2017, 2017, 9459156. [Google Scholar] [CrossRef]
- Arce-Sillas, A.; Alvarez-Luquin, D.D.; Tamaya-Dominguez, B.; Gomez-Fuentes, S.; Trejo-Garcia, A.; Melo-Salas, M.; Cardenas, G.; Rodriguez-Ramirez, J.; Adalid-Peralta, L. Regulatory T Cells: Molecular Actions on Effector Cells in Immune Regulation. J. Immunol. Res. 2016, 2016, 12. [Google Scholar] [CrossRef]
- Yang, S.; Yu, M. Role of Goblet Cells in Intestinal Barrier and Mucosal Immunity. J. Inflamm. Res. 2021, 14, 3171–3183. [Google Scholar] [CrossRef]
- Tang, C.; Ding, R.; Sun, J.; Liu, J.; Kan, J.; Jin, C. The impacts of natural polysaccharides on intestinal microbiota and immune responses–a review. Food Funct. 2019, 10, 2290–2312. [Google Scholar] [CrossRef] [PubMed]
- Caspary, W.F. Physiology and pathophysiology of intestinal absorption. Am. J. Clin. Nutr. 1992, 55, 299S–308S. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.M. The mechanics of crypt morphogenesis. Nat. Cell Biol. 2021, 23, 678–679. [Google Scholar] [CrossRef]
- Tian, H.; Liang, Y.; Liu, G.; Li, Y.; Deng, M.; Liu, D.; Guo, Y.; Sun, B. Moringa oleifera polysaccharides regulates caecal microbiota and small intestinal metabolic profile in C57BL/6 mice. Int. J. Biol. Macromol. 2021, 182, 595–611. [Google Scholar] [CrossRef]
- Feng, H.; Fan, J.; Lin, L.; Liu, Y.; Chai, D.; Yang, J. Immunomodulatory Effects of Phosphorylated Radix Cyathulae officinalis Polysaccharides in Immunosuppressed Mice. Molecules 2019, 24, 4150. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Ouyang, K.; Chen, H.; Yang, Z.; Hu, W.; Wang, N.; Liu, X.; Wang, W. Immunomodulatory effect of Cyclocarya paliurus polysaccharide in cyclophosphamide induced immunocompromised mice. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100224. [Google Scholar] [CrossRef]
- Yu, Z.M.; Huang, X.H.; Yan, C.Q.; Gao, J.; Liang, Z.S. Effect of Fuzheng Jiedu granule on immunological function and level of immune-related cytokines in immune-suppressed mice. J. Integr. Agric. 2016, 15, 650–657. [Google Scholar] [CrossRef]
- Fu, Y.P.; Feng, B.; Zhu, Z.K.; Feng, X.; Chen, S.F.; Li, L.X.; Yin, Z.Q.; Huang, C.; Chen, X.F.; Zhang, B.Z.; et al. The Polysaccharides from Codonopsis pilosula Modulates the Immunity and Intestinal Microbiota of Cyclophosphamide-Treated Immunosuppressed Mice. Molecules 2018, 23, 1801. [Google Scholar] [CrossRef]
- Waldmann, T.A. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 2006, 6, 595–601. [Google Scholar] [CrossRef]
- Smith, K.A.; Maizels, R.M. IL-6 controls susceptibility to helminth infection by impeding Th2 responsiveness and altering the Treg phenotype in vivo. Eur. J. Immunol. 2014, 44, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Ner-Gaon, H.; Varon, J.; Cullen, A.M.; Guo, J.; Choi, J.; Barragan-Bradford, D.; Higuera, A.; Pinilla-Vera, M.; Short, S.A.; et al. Post-sepsis immunosuppression depends on NKT cell regulation of mTOR/IFN-gamma in NK cells. J. Clin. Investig. 2020, 130, 3238–3252. [Google Scholar] [CrossRef] [PubMed]








| Group | Initial Weight (g) | Weight on the Fourth Day (g) | Final Weight (g) | Weight Gain (g) |
|---|---|---|---|---|
| NC | 18.14 ± 1.31 | 18.02 ± 0.95 * | 18.59 ± 1.00 | 0.55 ± 0.69 |
| MC | 19.28 ± 1.07 | 17.20 ± 1.02 # | 18.56 ± 0.73 | −0.72 ± 0.54 |
| AFPL | 18.63 ± 1.16 | 16.63 ± 1.15 # | 18.25 ± 0.69 | −0.04 ± 0.84 |
| AFPM | 18.44 ± 1.02 | 16.60 ± 1.40 # | 17.90 ± 0.82 | −0.17 ± 0.61 |
| AFPH | 18.63 ± 0.81 | 16.57 ± 0.93 # | 17.81 ± 0.88 | −0.48 ± 0.32 |
| PC | 19.29 ± 1.12 | 17.26 ± 0.87 # | 18.69 ± 1.04 | −0.38 ± 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, A.; Wan, H.; Feng, L.; Yang, B.; Wan, Y. Protective Effect of Anoectochilus formosanus Polysaccharide against Cyclophosphamide-Induced Immunosuppression in BALB/c Mice. Foods 2023, 12, 1910. https://doi.org/10.3390/foods12091910
Xie A, Wan H, Feng L, Yang B, Wan Y. Protective Effect of Anoectochilus formosanus Polysaccharide against Cyclophosphamide-Induced Immunosuppression in BALB/c Mice. Foods. 2023; 12(9):1910. https://doi.org/10.3390/foods12091910
Chicago/Turabian StyleXie, Anqi, Hao Wan, Lei Feng, Boyun Yang, and Yiqun Wan. 2023. "Protective Effect of Anoectochilus formosanus Polysaccharide against Cyclophosphamide-Induced Immunosuppression in BALB/c Mice" Foods 12, no. 9: 1910. https://doi.org/10.3390/foods12091910