Effect of Controlled Atmosphere Packaging on the Physiology and Quality of Fresh-Cut Dictyophora rubrovolvata
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Treatment and Storage
2.3. Decay Rate, Respiration Rate, Malondialdehyde (MDA) Content, Relative Conductivity, Ethanol Content, and Low-Field Nuclear Magnetic Resonance (LF-NMR) Transverse Relaxation Measurements
2.4. Texture, Browning Degree, Polyphenol Oxidase (PPO) Activity, Catalase (CAT) Activity, and Total Phenolic (TP) Content
2.5. Polysaccharides Content
2.6. Free Amino Acid
2.7. 5′-Nucleotides
2.8. Equivalent Umami Concentration (EUC)
2.9. Electronic Nose
2.10. Total Colony Numbers
2.11. Statistical Analysis
3. Results and Discussion
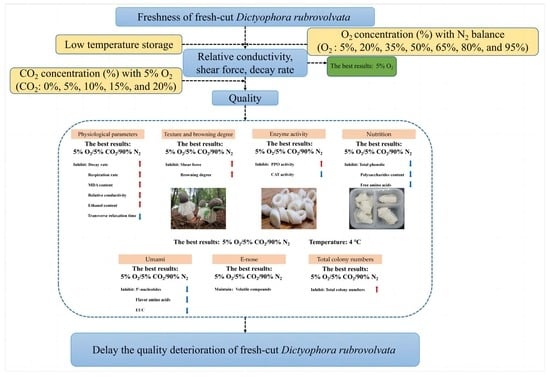
3.1. Effect of O2 Concentration (%) on the Shelf Life and Quality of Fresh-Cut D. rubrovolvata
3.2. Effect of CO2 Concentration (%) with 5% O2 on the Shelf Life of Fresh-Cut D. rubrovolvata
3.2.1. Changes in Physiological Parameters
3.2.2. Changes in Texture and Browning Degree
3.2.3. Changes in Enzyme Activity
3.2.4. Changes in Nutrition
3.2.5. Changes in Umami
3.2.6. E-Nose Analysis
3.2.7. Changes in Total Colony Numbers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Castellanos-Reyes, K.; Villalobos-Carvajal, R.; Beldarrain-Iznaga, T. Fresh mushroom preservation techniques. Foods 2019, 10, 2126. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.S.; Martins, A.; Vasconcelos, M.H.; Morales, P.; Ferreira, I.C.F.R. Functional foods based on extracts or compounds derived from mushrooms. Trends Food Sci. Technol. 2017, 66, 48–62. [Google Scholar] [CrossRef]
- Li, C.T.; Xu, S. Edible mushroom industry in China: Current state and perspectives. Appl. Microbiol. Biotechnol. 2022, 106, 3949–3955. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, J.; Huang, X.; Nie, S. Structural characteristics and rheological properties of high viscous glucan from fruit body of Dictyophora rubrovolvata. Food Hydrocoll. 2020, 101, 105514. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.; Zhao, J.; Tang, X.; Pasquali, M.; Migheli, Q.; Berg, G.; Cernava, T. Occurrence of green mold disease on Dictyophora rubrovolvata caused by Trichoderma koningiopsis. J. Plant Pathol. 2021, 103, 981–984. [Google Scholar] [CrossRef]
- Sun, L.; Bao, C.; Chang, W.; Zhuang, Y. Preparation, characterisation, antioxidant and antiglycation activities of the novel polysaccharides from the pileus of Dictyophora rubrovolvata. Int. J. Food Sci. Technol. 2016, 52, 161–170. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, L.; Yang, Z.; Bau, T.; Li, T.; Dai, Y. Resource diversity of Chinese macrofungi: Edible, medicinal and poisonous species. Fungal Divers. 2019, 98, 1–76. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Aguiló-Aguayo, I.; Martín-Belloso, O.; Soliva-Fortuny, R. Effects of pulsed light treatments on quality and antioxidant properties of fresh-cut mushrooms (Agaricus bisporus). Postharvest Biol. Technol. 2010, 56, 216–222. [Google Scholar] [CrossRef]
- Kumar, K.; Mehra, R.; Guine, R.P.F.; Lima, M.J.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible mushrooms: A comprehensive review on bioactive compounds with health benefits and processing aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef]
- Sun, T.; Bian, J.; Wang, Y.; Hu, J.; Yun, X.; Chen, E.; Dong, T. One-step synthesis of poly(L-lactic acid)-based soft films with gas permselectivity for white mushrooms (Agaricus bisporus) preservation. Foods 2023, 12, 586. [Google Scholar] [CrossRef]
- Falagan, N.; Terry, L. Recent advances in controlled and modified atmosphere of fresh produce postharvest technologies to reduce food waste and maintain fresh produce quality. Johns. Matthey Technol. Rev. 2018, 62, 107–117. [Google Scholar] [CrossRef]
- Amodio, M.; Colelli, G. Controlled-atmosphere storage of fresh-cut ‘cardoncello’ mushrooms (Pleurotus eryngii). Acta Hortic. 2003, 599, 731–735. [Google Scholar] [CrossRef]
- Hong, G.; Crisosto, C.; Cantwell, M. Quality and physiology of two cultivars of fresh-cut figs in relation to ripeness, storage temperature and controlled atmosphere. Acta Hortic. 2016, 1141, 213–219. [Google Scholar] [CrossRef]
- Esmaeili, Y.; Zamindar, N.; Paidari, S.; Ibrahim, S.A.; Nafchi, A.M. The synergistic effects of aloe vera gel and modified atmosphere packaging on the quality of strawberry fruit. J. Food Process. Preserv. 2021, 45, e16003. [Google Scholar] [CrossRef]
- Li, L.; Kitazawa, H.; Wang, X.; Sun, H. Regulation of respiratory pathway and electron transport chain in relation to senescence of postharvest white mushroom (Agaricus bisporus) under high O2/CO2 controlled atmospheres. J. Agric. Food Chem. 2017, 65, 3352–3360. [Google Scholar] [CrossRef]
- Li, L.; Sun, H.; Kitazawa, H.; Wang, X. Effects of a high O2 dynamic-controlled atmosphere technology on the browning of postharvest white mushroom (Agaricus bisporus) in relation to energy metabolism. Food Sci. Technol. Int. 2017, 23, 385–395. [Google Scholar] [CrossRef]
- Wang, L.; Guo, Y.; Wang, X.; Zhang, X. Short-term O2/CO2 controlled atmosphere altered the water status and thus promoted phenolic biosynthesis during wound healing of fresh-cut white mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2022, 188, 111879. [Google Scholar] [CrossRef]
- Wang, L.; Han, M.; Cui, Y.; Wang, X.; Shang, X.; Wang, C. Pretreatment with high oxygen controlled atmosphere enhanced fresh-cut white mushroom (Agaricus bisporus) quality via activating wounding stress defenses. J. Sci. Food Agric. 2022, 102, 3359–3369. [Google Scholar] [CrossRef]
- Rux, G.; Mahajan, P.V.; Geyer, M.; Linke, M.; Pant, A.; Saengerlaub, S.; Caleb, O.J. Application of humidity-regulating tray for packaging of mushrooms. Postharvest Biol. Technol. 2015, 108, 102–110. [Google Scholar] [CrossRef]
- Zhang, Y.; Duo, Y.; Yan, R.; Xu, Y.; Xing, M.; Liao, S.; Wan, C.; Chen, C.; Zhu, L.; Kai, W.; et al. Amelioration of chilling injury by fucoidan in cold-stored cucumber via membrane lipid metabolism regulation. Foods 2023, 12, 301. [Google Scholar] [CrossRef]
- Huang, Q.; Qian, X.; Jiang, T.; Zheng, X. Effect of chitosan and guar gum based composite edible coating on quality of mushroom (Lentinus edodes) during postharvest storage. Sci. Hortic. 2019, 253, 382–389. [Google Scholar] [CrossRef]
- He, Y.; Fan, G.; Wu, C.; Kou, X.; Li, T.; Tian, F.; Gong, H. Influence of packaging materials on postharvest physiology and texture of garlic cloves during refrigeration storage. Food Chem. 2019, 298, 125019. [Google Scholar] [CrossRef]
- Ventura-Aguilar, R.I.; Colinas-León, M.T.; Bautista-Baños, S. Combination of sodium erythorbate and citric acid with MAP, extended storage life of sliced oyster mushrooms. LWT—Food Sci. Technol. 2017, 79, 437–444. [Google Scholar] [CrossRef]
- Cheng, S.; Ranran, L.; Yang, H.; Wang, S.; Lin, R.; Tian, M. Characterisation of moisture migration of shiitake mushroom (Lentinula edodes) during storage and its relationship to quality deterioration. Int. J. Food Sci. Technol. 2019, 55, 2132–2140. [Google Scholar] [CrossRef]
- Jahanbakhshi, A.; Abbaspour-Gilandeh, Y.; Gundoshmian, T.M. Determination of physical and mechanical properties of carrot in order to reduce waste during harvesting and post-harvesting. Food Sci. Nutr. 2018, 6, 1898–1903. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Zhang, X.; Kan, J.; Jin, C. Effect of gallic acid grafted chitosan film packaging on the postharvest quality of white button mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2019, 147, 39–47. [Google Scholar] [CrossRef]
- Plesoianu, A.M.; Nour, V. Effect of some polysaccharide-based edible coatings on fresh white button mushroom (Agaricus bisporus) quality during cold storage. Agriculture 2022, 12, 1491. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, X.; Yin, J.; Nie, S. Bioactive polysaccharide from edible Dictyophora spp.: Extraction, purification, structural features and bioactivities. Bioact. Carbohydr. Diet. Fibre 2018, 14, 25–32. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Li, Z.; Wu, W.; Tang, X. Analysis and evaluation of the characteristic taste components in portobello mushroom. J. Food Sci. 2018, 83, 1542–1551. [Google Scholar] [CrossRef]
- Pei, F.; Yang, W.; Ma, N.; Fang, Y.; Zhao, L.; An, X.; Xin, Z.; Hu, Q. Effect of the two drying approaches on the volatile profiles of button mushroom (Agaricus bisporus) by headspace GC–MS and electronic nose. LWT—Food Sci. Technol. 2016, 72, 343–350. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, M.; Devahastin, S.; Guo, Z. Effects of pressurized argon and nitrogen treatments in combination with modified atmosphere on quality characteristics of fresh-cut potatoes. Postharvest Biol. Technol. 2019, 149, 159–165. [Google Scholar] [CrossRef]
- Li, P.; Zhang, X.; Hu, H.; Sun, Y.; Wang, Y.; Zhao, Y. High carbon dioxide and low oxygen storage effects on reactive oxygen species metabolism in Pleurotus eryngii. Postharvest Biol. Technol. 2013, 85, 141–146. [Google Scholar] [CrossRef]
- Lyn, F.H.; Adilah, Z.A.M.; Nor-Khaizura, M.A.R.; Jamilah, B.; Hanani, Z.A.N. Application of modified atmosphere and active packaging for oyster mushroom (Pleurotus ostreatus). Food Packag. Shelf Life 2020, 23, 100451. [Google Scholar] [CrossRef]
- Capotorto, I.; Innamorato, V.; Cefola, M.; Cervellieri, S.; Lippolis, V.; Longobardi, F.; Logrieco, A.F.; Pace, B. High CO2 short-term treatment to preserve quality and volatiles profile of fresh-cut artichokes during cold storage. Postharvest Biol. Technol. 2020, 160, 111056. [Google Scholar] [CrossRef]
- Wang, H.; Xu, N.; Peng, Y.; Zhang, X. The determination of low oxygen threshold in garlic scapes during controlled atmosphere storage. J. Sci. Food Agric. 2022, 102, 4949–4954. [Google Scholar] [CrossRef]
- Wang, Y.; Mo, Y.; Li, D.; Xiang, C.; Jiang, Z.; Wang, J. The main factors inducing postharvest lignification in king oyster mushrooms (Pleurotus eryngii): Wounding and ROS-mediated senescence. Food Chem. 2019, 301, 125224. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Li, J.; Han, X.; Zhang, L.; Jiang, T.; Xia, M. Effects of active modified atmosphere packaging on postharvest quality of shiitake mushrooms (Lentinula edodes) stored at cold storage. J. Integr. Agric. 2012, 11, 474–482. [Google Scholar] [CrossRef]
- Harada-Padermo, S.; Dias-Faceto, L.; Selani, M.; Conti-Silva, A.; Vieira, T. Umami ingredient, a newly developed flavor enhancer from shiitake byproducts, in low-sodium products: A study case of application in corn extruded snacks. LWT—Food Sci. Technol. 2021, 138, 110806. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, M.; Adhikari, B.; Guo, Z. Effect of ultrasound combined with controlled atmosphere on postharvest storage quality of cucumbers (Cucumis sativus L.). Food Bioprocess Technol. 2018, 11, 1328–1338. [Google Scholar] [CrossRef]
- Waghmare, R.B.; Annapure, U.S. Integrated effect of sodium hypochlorite and modified atmosphere packaging on quality and shelf life of fresh-cut cilantro. Food Packag. Shelf Life 2015, 3, 62–69. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Z.; Wang, R.; Ma, C.; Li, J.; Lei, J.; Ji, N.; Pan, X.; Chen, T. Effect of Controlled Atmosphere Packaging on the Physiology and Quality of Fresh-Cut Dictyophora rubrovolvata. Foods 2023, 12, 1665. https://doi.org/10.3390/foods12081665
Xia Z, Wang R, Ma C, Li J, Lei J, Ji N, Pan X, Chen T. Effect of Controlled Atmosphere Packaging on the Physiology and Quality of Fresh-Cut Dictyophora rubrovolvata. Foods. 2023; 12(8):1665. https://doi.org/10.3390/foods12081665
Chicago/Turabian StyleXia, Ziqian, Rui Wang, Chao Ma, Jiangkuo Li, Jiqing Lei, Ning Ji, Xianxing Pan, and Tongjie Chen. 2023. "Effect of Controlled Atmosphere Packaging on the Physiology and Quality of Fresh-Cut Dictyophora rubrovolvata" Foods 12, no. 8: 1665. https://doi.org/10.3390/foods12081665