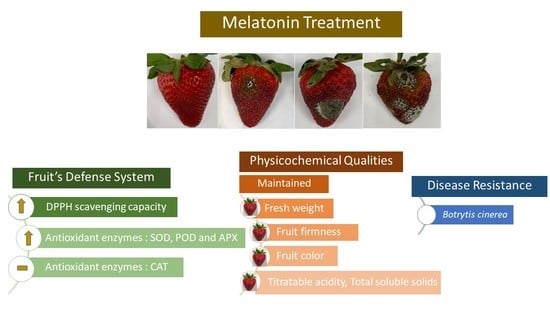
Melatonin Treatment of Strawberry Fruit during Storage Extends Its Post-Harvest Quality and Reduces Infection Caused by Botrytis cinerea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Material
2.2. Exogenous Melatonin (MT) Treatment and Botrytis cinerea Artificial Inoculation
2.3. Disease Assessment
2.4. qPCR Quantification of B. cinerea Biomass in Strawberry Fruit
2.5. Physicochemical Quality Measurements
2.6. Antioxidant Capacity Measurement
2.7. Antioxidant Enzyme Activity Measurement
2.8. Statistical Analysis
3. Results
3.1. Visual Appearance and Disease Development
3.2. Physicochemical Qualities: Fruit Colour, TSS, TA, Fresh Weight Loss, and Fruit Firmness
3.3. Antioxidant Capacity and Antioxidant Enzymes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tulipani, S.; Marzban, G.; Herndl, A.; Laimer, M.; Mezzetti, B.; Battino, M. Influence of environmental and genetic factors on health-related compounds in strawberry. Food Chem. 2011, 124, 906–913. [Google Scholar] [CrossRef]
- National Agricultural Statistics Service (NASS). Economics, Statistics and Market Information System. 2021. Available online: https://usda.library.cornell.edu/concern/publications/zs25x846c?locale=en (accessed on 6 June 2022).
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; VanKan, J.A.L. Botrytis cinerea: The causeof grey mold disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, L.E.; Page-Weir, N.E.M.; Chagan, A.; Brash, D.W.; Klementz, D.; Bycroft, B.L.; Connolly, P.G.; Waddell, B.C.; Gilbertson, R.; Bollen, F.; et al. Phosphine fumigation to disinfest kiwifruit. NZ Plant Prot. 2012, 65, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiter, R.J.; Tan, D.X.; Zhou, Z.; Cruz, M.H.; Fuentes-Broto, L.; Galano, A. Phytomelatonin: Assisting plants to survive and thrive. Molecules 2015, 20, 7396–7437. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Exp. Bot. 2020, 63, 126–145. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.Y.; Shi, X.Y.; Xiao, H.; Kang, K.; Liu, X.Y.; Zhan, J.C.; Huang, W.D. Effect of cultivar, temperature, and environment conditions on thedynamic change of melatonin in mulberry fruit development and wine fermentation. J. Food Sci. 2016, 81, 958–967. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, N.; Wang, J.; Zhang, H.; Li, D.; Shi, J.; Li, R.; Weeda, S.; Zhao, B.; Ron, S.; et al. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J. Exp. Bot. 2015, 66, 657–668. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Yang, H.; Tie, W.W.; Yan, Y.; Ding, Z.H.; Liu, Y. Natural variationin banana varieties highlights the role of melatonin in post-harvest ripening and quality. J. Agric. Food Chem. 2017, 65, 9987–9994. [Google Scholar] [CrossRef]
- Xu, Y.; Charles, M.T.; Luo, Z.; Mimee, B.; Tong, Z.; Veronneau, P.Y.; Roussel, D.; Rolland, D. Ultraviolet-C primming of strawberry leaves against subsequent Mycosphaerella fragariac infection involves the action of reactive oxygen species, plant hormones and terpenes. Plant Cell Environ. 2019, 42, 815–831. [Google Scholar] [CrossRef]
- Lin, Y.; Fan, L.; Xia, X.; Wang, Z.; Yin, Y.; Cheng, Y.; Li, Z. Melatonin decreases resistance to postharvest green mold on citrus fruit by scavenging defense-relatedreactive oxygen species. Postharvest Biol. Technol. 2019, 153, 21–30. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of melatonin treatment on thepostharvest quality of strawberry fruit. Postharvest Biol. Technol. 2018, 139, 47–55. [Google Scholar] [CrossRef]
- Yin, L.; Wang, P.; Li, M.; Ke, X.; Li, C.; Liang, D.; Xu, S.; Ma, X.; Li, C.; Zou, Y.; et al. Exogenous melatonin improves Malus resistance to Marssonina apple blotch. J. Pineal Res. 2013, 54, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yu, Z.; Zhang, Y.; Li, B.; Liang, W.; Wang, C. Control efficiency ofexogenous melatonin against postharvest apple grey mold and its influence on theactivity of defensive enzymes. Plant Physiol. 2017, 53, 1753–1760. [Google Scholar]
- Gao, S.; Ma, W.; Lyu, X.; Cao, X.; Yao, Y. Melatonin may increase diseaseresistance and flavonoid biosynthesis through effects on DNA methylation and geneexpression in grape berries. BMC Plant Biol. 2020, 20, 231. [Google Scholar] [CrossRef]
- Li, T.; Wu, Q.; Zhu, H.; Zhou, Y.; Jiang, Y.; Gao, H.; Yun, Z. Comparative transcriptomicand metabolic analysis reveals the effect of melatonin on delaying anthracnoseincidence upon postharvest banana fruit peel. BMC Plant Biol. 2019, 19, 289. [Google Scholar] [CrossRef]
- Hu, M.; Li, J.; Rao, J. Effect of melatonin on ripening and senescence of postharvest kiwifruits. Food Sci. 2018, 39, 226–232. [Google Scholar]
- Gao, H.; Zhang, Z.K.; Chai, H.K.; Cheng, N.; Yang, Y.; Wang, D.N. Melatonin treatment delays postharvest senescence and regulates reactive oxygenspecies metabolism in peach fruit. Postharvest Biol. Technol. 2016, 118, 103–110. [Google Scholar] [CrossRef]
- Testempasis, S.; Tanou, G.; Minas, I.; Samiotaki, M.; Molassiotis, A.; Karaoglanidis, G. Unraveling interactions of the necrotrophic fungal species Botrytis cinerea with 1methylcyclopropene or ozone-Treated apple fruit using proteomic analysis. Front. Plant Sci. 2021, 12, 644255.21. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bullet. 1987, 19, 11–15. [Google Scholar]
- AOAC. Official Methods of Analysis, 16th ed.; Association of OfficialAnalytical Chemists International: Washington, DC, USA, 1995. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical methodto evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Suiubon, S.; Supapvanich, S.; Promyou, S. Postharvest quality maintenance oflongan fruit by ultraviolet-C incorporated with salicylic acid application. Emir. J. Food Agric. 2017, 29, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation ofmicrogram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Fard, J.R. Melatonin treatment attenuates postharvest decayand maintains nutrition quality of strawberry fruit (Fragaria x anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem. 2017, 221, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Bal, E. Physicochemical changes in ‘Santa Rosa’ plum fruit treated withmelatonin during cold storage. J. Food Meas. Charact. 2019, 13, 1713–1720. [Google Scholar] [CrossRef]
- Lee, H.Y.; Byeon, Y.; Tan, D.X.; Reiter, R.J.; Back, K. Arabidopsis serotoninN-acetyltransferase knockout mutant plants exhibit decreased melatonin andsalicylic acid levels resulting in susceptibility to an avirulent pathogen. J. Pineal Res. 2015, 58, 291–299. [Google Scholar] [CrossRef]
- Liu, S.; Huang, H.; Huber, D.J.; Pan, Y.; Shi, X.; Zhang, Z. Delay of ripening and softeningin ‘Guifei’ mango fruit by postharvest application of melatonin. Postharvest Biol.Technol. 2020, 163, 111136. [Google Scholar] [CrossRef]
- Tijero, V.; Munoz, P.; Munne-Bosch, S. Melatonin as an inhibitor of sweet cherriesripening in orchard trees. Plant Physiol. Biochem. 2019, 140, 88–95. [Google Scholar] [CrossRef]
- Onik, J.C.; Wai, S.C.; Li, A.; Lin, Q.; Sun, Q.; Wang, Z.; Duan, Y. Melatonin treatmentreduces ethylene production and maintains fruit quality in apple during postharveststorage. Food Chem. 2020, 337, 127753. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Zhang, H.; Cong, L.; Zhai, R.; Yang, C.; Wang, Z.; Ma, Z.; Xu, L. Melatonininhibits ethylene synthesis via nitric oxide regulation to delay postharvest senescencein pears. J. Agric. Food Chem. 2019, 67, 2279–2288. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Yang, Q.; Zhao, Q. Exogenous melatonin delays postharvest fruitsenescence and maintains the quality of sweet cherries. Food Chem. 2019, 301, 125–311. [Google Scholar] [CrossRef]
- Jiang, Y.; Duan, X.; Joyce, D.; Zhang, Z.; Li, J. Advances in understanding of enzymatic browning in harvested litchi fruit. Food Chem. 2004, 88, 443–446. [Google Scholar] [CrossRef]
- Jannatizadeh, A. Exogenous melatonin applying confers chilling tolerance inpomegranate fruit during storage. Sci. Hortic. 2019, 246, 544–549. [Google Scholar] [CrossRef]
- Rastegar, S.; Khankandani, H.H.; Rahimzadeh, M. Effects of melatonin treatment onthe biochemical changes and antioxidant enzyme activity of mango fruit duringstorage. Sci. Hortic. 2020, 259, 108835. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Day after Treatment | Treatments | Color § | TSS (°Brix) | TA (% Citric Acid) | ||
|---|---|---|---|---|---|---|
| L* | a* | b* | ||||
| Day 0 | 30.48 a | 22.68 c | 23.67 b | 6.14 c | 1.02 a | |
| Day 2 | Inoculated control (+B. cinerea) | 24.69 c | 26.32 a | 23.99 b | 6.02 c | 0.72 b |
| Positive control (NaOCl) | 25.89 c | 26.96 a | 24.60 b | 6.32 c | 0.70 b | |
| Control | 25.53 c | 26.22 a | 25.35 a | 6.56 c | 0.65 b | |
| Inoculated positive control (NaOCl + B. cinerea) | 24.49 c | 26.48 a | 25.29 a | 6.82 c | 0.63 b | |
| Melatonin | 27.16 b | 23.53 b | 24.30 b | 6.54 c | 0.78 b | |
| Melatonin + B. cinerea | 26.19 b | 23.24 b | 24.00 b | 6.22 c | 0.71 b | |
| Day 4 | Inoculated control (+B. cinerea) | 22.30 d | 26.91 a | 23.95 b | 8.00 a | 0.68 b |
| Positive control (NaOCl) | 24.70 c | 26.75 a | 24.49 b | 7.42 b | 0.66 b | |
| Control | 23.55 d | 27.21 a | 25.53 a | 8.10 a | 0.60 b | |
| Inoculated positive control (NaOCl + B. cinerea) | 24.45 c | 26.44 a | 25.37 a | 7.86 b | 0.59 b | |
| Melatonin | 26.55 b | 24.56 b | 24.70 b | 7.64 b | 0.64 b | |
| Melatonin + B. cinerea | 26.96 b | 24.79 b | 24.50 b | 7.38 b | 0.68 b | |
| Day 6 | Inoculated control (+B. cinerea) | 19.74 e | 28.54 a | 25.15 a | 8.30 a | 0.50 c |
| Positive control (NaOCl) | 23.19 d | 26.65 a | 25.64 a | 7.46 b | 0.51 c | |
| Control | 22.09 d | 28.67 a | 26.57 a | 8.22 a | 0.55 c | |
| Inoculated positive control (NaOCl + B. cinerea) | 22.24 d | 28.54 a | 25.77 a | 7.76 b | 0.53 c | |
| Melatonin | 25.55 c | 25.36 b | 25.35 a | 7.32 b | 0.61 b | |
| Melatonin + B. cinerea | 25.65 c | 25.74 b | 26.59 a | 7.98 b | 0.68 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Promyou, S.; Raruang, Y.; Chen, Z.-Y. Melatonin Treatment of Strawberry Fruit during Storage Extends Its Post-Harvest Quality and Reduces Infection Caused by Botrytis cinerea. Foods 2023, 12, 1445. https://doi.org/10.3390/foods12071445
Promyou S, Raruang Y, Chen Z-Y. Melatonin Treatment of Strawberry Fruit during Storage Extends Its Post-Harvest Quality and Reduces Infection Caused by Botrytis cinerea. Foods. 2023; 12(7):1445. https://doi.org/10.3390/foods12071445
Chicago/Turabian StylePromyou, Surassawadee, Yenjit Raruang, and Zhi-Yuan Chen. 2023. "Melatonin Treatment of Strawberry Fruit during Storage Extends Its Post-Harvest Quality and Reduces Infection Caused by Botrytis cinerea" Foods 12, no. 7: 1445. https://doi.org/10.3390/foods12071445