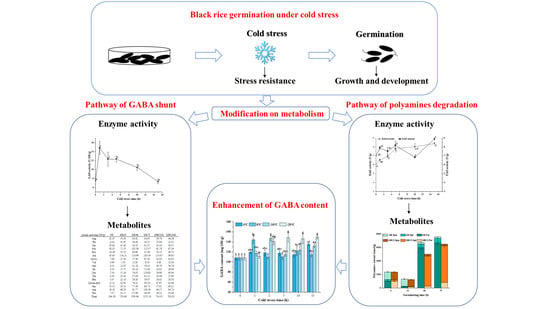
Accelerated Accumulation of γ-Aminobutyric Acid and Modifications on Its Metabolic Pathways in Black Rice Grains by Germination under Cold Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Controlled Germination
2.3. Cold Stress Treatment and Experimental Design
- Before germination, black rice grains were placed in a refrigerator at 4, 0, −10, and −20 °C for 1, 3, 5, 10, and 15 h, respectively. The GABA content was measured after 24 h of germination.
- To investigate the influence of germination time (24, 36, 48, 60, and 72 h) on the content of GABA that had been subjected to cold stress, the stress time with the maximum GABA production at each temperature was chosen.
- For follow-up investigations, the sample with excellent GABA accumulation after Step 1 or 2 was chosenand germinated black rice grains that had not been subjected to cold stress were utilized as a control.
2.4. Determination of GABA Content
2.5. Measurement of Glutamate Decarboxylase (GAD) Activity
2.6. Cytochemical Localization of Ca2+ in Germ Cells
2.7. Measurement of γ-. Aminobutyric Acid Aminotransferase (GABA-T) Activity
2.8. Measurement of Protease Activity
2.9. Determination of Protein Content and Amino Acids
2.10. Measurement of Diamine Oxidase (DAO) and Polyamine Oxidase (PAO) Activity
2.11. Determination of Polyamines Content
2.12. Measurement of Amino-Aldehyde Dehydrogenase (AMADH) Activity
2.13. Statistical Analysis
3. Results and Discussion
3.1. GABA Content
3.2. GAD Activity and Distribution of Ca2+ in Germ Cells
3.3. GABA-T Activity
3.4. Protease Activity, Protein Content, and Free Amino Acids
3.5. DAO and PAO Activity and Polyamines Content
3.6. AMADH activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, G.; Chen, X.; Wang, N.; Tian, J.; Song, S.; Wu, X.; Wang, L.; Wen, C. Effect of konjac glucomannan with different viscosities on the quality of surimi-wheat dough and noodles. Int. J. Biol. Macromol. 2022, 221, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q. Purple Tomatoes, Black Rice and Food Security. Nat. Rev. Genet. 2021, 22, 414. [Google Scholar] [CrossRef]
- Moirangthem, K.; Jenkins, D.; Ramakrishna, P.; Rajkumari, R.; Cook, D. Indian black rice: A brewing raw material with novel functionality. J. Inst. Brew. 2020, 126, 35–45. [Google Scholar] [CrossRef]
- Brickley, S.G.; Mody, I. Extrasynaptic GABA(A) Receptors: Their Function in the CNS and Implications for Disease. Neuron 2012, 73, 23–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luscher, B.; Shen, Q.; Sahir, N. The GABAergic deficit hypothesis of major depressive disorder. Mol. Psychiatry 2011, 16, 383–406. [Google Scholar] [CrossRef] [Green Version]
- Moehler, H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology 2012, 62, 42–53. [Google Scholar] [CrossRef]
- Stagg, C.J.; Bachtiar, V.; Johansen-Berg, H. The Role of GABA in Human Motor Learning. Curr. Biol. 2011, 21, 480–484. [Google Scholar] [CrossRef] [Green Version]
- Cho, D.H.; Lim, S.T. Germinated brown rice and its bio-functional compounds. Food Chem. 2016, 196, 259–271. [Google Scholar] [CrossRef]
- Soa, A.; Ceca, B.; Sob, C.; Ure, A.; Afm, A.; Aas, A.; Sso, D.; Nd, E.; Oaa, F. Impact of germination alone or in combination with solid-state fermentation on the physicochemical, antioxidant, in vitro digestibility, functional and thermal properties of brown finger millet flours. LWT 2021, 154, 112734. [Google Scholar]
- Luo, X.; Li, D.; Tao, Y.; Wang, P.; Yang, R.; Han, Y. Effect of static magnetic field treatment on the germination of brown rice: Changes in α-amylase activity and structural and functional properties in starch. Food Chem. 2022, 383, 132392. [Google Scholar] [CrossRef]
- Wei, Y.; Shohag, M.J.I.; Ying, F.; Yang, X.; Wu, C.; Wang, Y. Effect of ferrous sulfate fortification in germinated brown rice on seed iron concentration and bioavailability. Food Chem. 2013, 138, 1952–1958. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Hwang, I.G.; Kim, T.M.; Woo, K.S.; Park, D.S.; Kim, J.H.; Kim, D.J.; Lee, J.; Lee, Y.R.; Jeong, H.S. Chemical and functional components in different parts of rough rice (Oryza sativa L.) before and after germination. Food Chem. 2012, 134, 288–293. [Google Scholar] [CrossRef]
- Baranzelli, J.; Kringel, D.H.; Colussi, R.; Paiva, F.F.; Aranha, B.C.; de Miranda, M.Z.; Zavareze, E.D.R.; Guerra Dias, A.R. Changes in enzymatic activity, technological quality and gamma-aminobutyric acid (GABA) content of wheat flour as affected by germination. LWT-Food Sci. Technol. 2018, 90, 483–490. [Google Scholar] [CrossRef]
- Ding, J.; Hou, G.G.; Nemzer, B.V.; Xiong, S.; Dubat, A.; Feng, H. Effects of controlled germination on selected physicochemical and functional properties of whole-wheat flour and enhanced gamma-aminobutyric acid accumulation by ultrasonication. Food Chem. 2018, 243, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Kim, H.S.; Lim, S.T.; Reddy, C.K. Enhanced accumulation of gamma-aminobutyric acid in rice bran using anaerobic incubation with various additives. Food Chem. 2019, 271, 187–192. [Google Scholar] [CrossRef]
- Wattananapakasem, I.; Penjumras, P.; Malaithong, W.; Nawong, S.; Poomanee, W.; Kinoshita, H. Effect of heat–moisture treatment of germinated black rice on the physicochemical properties and its utilization by lactic acid bacteria. J. Food Sci. Technol. 2021, 58, 4636–4645. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiang, J.; Zhang, L.; Zhu, X.; Evers, J.; van der Werf, W.; Duan, L. Optimizing soaking and germination conditions to improve gamma-aminobutyric acid content in japonica and indica germinated brown rice. J. Funct. Foods 2014, 10, 283–291. [Google Scholar] [CrossRef]
- de Vasconcelos, M.W.P.L.; Menguer, P.K.; Hu, Y.; Revers, L.F.; Sperotto, R.A. Stress Signaling Responses in Plants. BioMed Res. Int. 2016, 4720280. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Aliniaeifard, S.; Hassani, B.; Niknam, V.; Lastochkina, O. Diverse role of gamma-aminobutyric acid in dynamic plant cell responses. Plant Cell Rep. 2019, 38, 847–867. [Google Scholar] [CrossRef]
- Khan, M.; Jalil, S.U.; Chopra, P.; Chhillar, H.; Ansari, M.I. Role of GABA in plant growth, development and senescence. Plant Gene 2021, 26, 100283. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Y.; Zhang, M.; Meng, W.; Xie, X.; Chen, Y. Cold atmospheric plasma treatment improves the γ-aminobutyric acid content of buckwheat seeds providing a new anti-hypertensive functional ingredient. Food Chem. 2022, 388, 133064. [Google Scholar]
- Yang, R.; Hui, Q.; Feng, X.; Feng, L.; Gu, Z.; Wang, P. The mechanism of freeze-thawing induced accumulation of gamma-aminobutyric acid in germinated soybean. J. Sci. Food Agric. 2020, 100, 1099–1105. [Google Scholar] [CrossRef]
- Khwanchai, P.; Chinprahast, N.; Pichyangkura, R.; Chaiwanichsiri, S. Gamma-aminobutyric acid and glutamic acid contents, and the GAD activity in germinated brown rice (Oryza sativa L.): Effect of rice cultivars. Food Sci. Biotechnol. 2014, 23, 373–379. [Google Scholar] [CrossRef]
- Du, W.; Nan, X.; Li, M. Effects of tutin and its derivatives on GAD and GABA-T in Pseudelatia separata (Lepidoptera: Noctuidae). Rev. Colomb. Entomol. 2012, 38, 81–86. [Google Scholar] [CrossRef]
- Li, C.J.; Cao, X.H.; Gu, Z.X.; Wen, H.B. A preliminary study of the protease activities in germinating brown rice (Oryza sativa L.). J. Sci. Food Agric. 2011, 91, 915–920. [Google Scholar] [CrossRef]
- Su, G.X.; An, Z.F.; Zhang, W.H.; Liu, Y.L. Light promotes the synthesis of lignin through the production of H2O2 mediated by diamine oxidases in soybean hypocotyls. J. Plant Physiol. 2005, 162, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Petrivalsky, M.; Brauner, F.; Luhova, L.; Gagneul, D.; Sebela, M. Aminoaldehyde dehydrogenase activity during wound healing of mechanically injured pea seedlings. J. Plant Physiol. 2007, 164, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.A.; Xiang, C.; Farooq, M.; Muhammad, N.; Yan, Z.; Hui, X.; Ke, Y.; Bruno, A.K.; Zhang, L.; Li, J. Cold Stress in Wheat: Plant Acclimation Responses and Management Strategies. Front. Plant Sci. 2021, 12, 676884. [Google Scholar] [CrossRef]
- Janska, A.; Marsik, P.; Zelenkova, S.; Ovesna, J. Cold stress and acclimation—What is important for metabolic adjustment? Plant Biology 2010, 12, 395–405. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saikusa, T.; Horino, T.; Mori, Y. Accumulation of γ-Aminobutyric Acid (Gaba) in the Rice Germ during Water Soaking. Biosci. Biotechnol. Biochem. 2014, 58, 2291–2292. [Google Scholar] [CrossRef]
- Akihiro, T.; Koike, S.; Tani, R.; Tominaga, T.; Watanabe, S.; Iijima, Y.; Aoki, K.; Shibata, D.; Ashihara, H.; Matsukura, C.; et al. Biochemical mechanism on GABA accumulation during fruit development in tomato. Plant Cell Physiol. 2008, 49, 1378–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che-Othman, M.H.; Jacoby, R.P.; Millar, A.H.; Taylor, N.L. Wheat mitochondrial respiration shifts from the tricarboxylic acid cycle to the GABA shunt under salt stress. New Phytol. 2020, 225, 1166–1180. [Google Scholar] [CrossRef]
- Michaeli, S.; Fait, A.; Lagor, K.; Nunes-Nesi, A.; Grillich, N.; Yellin, A.; Bar, D.; Khan, M.; Fernie, A.R.; Turano, F.J.; et al. A mitochondrial GABA permease connects the GABA shunt and the TCA cycle, and is essential for normal carbon metabolism. Plant J. 2011, 67, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Mazzucotelli, E.; Tartari, A.; Cattivelli, L.; Forlani, G. Metabolism of gamma-aminobutyric acid during cold acclimation and freezing and its relationship to frost tolerance in barley and wheat. J. Exp. Bot. 2006, 57, 3755–3766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimajiri, Y.; Oonishi, T.; Ozaki, K.; Kainou, K.; Akama, K. Genetic manipulation of the gamma-aminobutyric acid (GABA) shunt in rice: Overexpression of truncated glutamate decarboxylase (GAD2) and knockdown of gamma-aminobutyric acid transaminase (GABA-T) lead to sustained and high levels of GABA accumulation in rice kernels. Plant Biotechnol. J. 2013, 11, 594–604. [Google Scholar]
- Hildebrandt, T.M.; Nunes Nesi, A.; Araujo, W.L.; Braun, H.P. Amino Acid Catabolism in Plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [Green Version]
- Soni, S.; Kumar, A.; Sehrawat, N.; Kumar, N.; Kaur, G.; Kumar, A.; Mann, A. Variability of durum wheat genotypes in terms of physio-biochemical traits against salinity stress. Cereal Res. Commun. 2021, 49, 45–54. [Google Scholar] [CrossRef]
- Podlesakova, K.; Ugena, L.; Spichal, L.; Dolezal, K.; De Diego, N. Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. New Biotechnol. 2019, 48, 53–65. [Google Scholar] [CrossRef]
- Tiburcio, A.F.; Altabella, T.; Bitrian, M.; Alcazar, R. The roles of polyamines during the lifespan of plants: From development to stress. Planta 2014, 240, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kusano, T.; Berberich, T.; Tateda, C.; Takahashi, Y. Polyamines: Essential factors for growth and survival. Planta 2008, 228, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Su, G.X.; Zhang, W.H.; Liu, Y.L. Involvement of hydrogen peroxide generated by polyamine oxidative degradation in the development of lateral roots in soybean. J. Integr. Plant Biol. 2006, 48, 426–432. [Google Scholar] [CrossRef]
- Wang, K.; Xu, F.; Cao, S.; Wang, H.; Wei, Y.; Shao, X.; Zhou, W.; Zheng, Y. Effects of exogenous calcium chloride (CaCl2) and ascorbic acid (AsA) on the γ-aminobutyric acid (GABA) metabolism in shredded carrots. Postharvest Biol. Technol. 2019, 152, 111–117. [Google Scholar] [CrossRef]












| Amino Acid (mg/100 g) | NGB | GB-24 | GB-48 | GB-72 | GBCS-24 | GBCS-48 | GBCS-72 |
|---|---|---|---|---|---|---|---|
| Asp | 25.07 a | 54.92 c | 63.61 d | 44.91 b | 56.76 c | 46.28 b | 42.13 b |
| Thr | 4.34 a | 32.67 c | 36.44 c | 43.31 d | 27.84 b | 25.22 b | 25.45 b |
| Ser | 10.62 a | 41.82 cd | 43.52 d | 61.37 e | 35.43 b | 39.51 c | 42.67 d |
| Glu | 30.31 a | 71.52 c | 102.39 e | 112.37 f | 61.78 b | 87.34 d | 96.01 e |
| Gly | 16.29 a | 53.55 e | 49.90 d | 47.69 d | 53.55 e | 40.73 c | 34.73 b |
| Ala | 50.63 a | 116.21 c | 116.99 c | 183.59 d | 114.67 c | 99.93 b | 129.23 d |
| (Cys)2 | 7.46 a | 37.43 c | 57.36 d | 87.92 e | 42.62 c | 42.95 c | 26.48 b |
| Val | 2.68 b | 1.52 a | 12.81 d | 8.56 c | 9.63 c | 12.93 d | 30.94 e |
| Met | 4.21 a | 12.97 b | 41.18 d | 59.35 f | 26.70 c | 36.79 d | 50.00 e |
| Ile | 3.33 a | 15.71 b | 30.14 d | 51.64 f | 22.81 c | 29.09 d | 36.97 e |
| Leu | 7.34 a | 51.24 b | 76.05 d | 128.82 e | 58.89 c | 63.94 c | 84.73 d |
| Tyr | 3.34 a | 25.41 b | 37.03 c | 61.21 d | 23.49 b | 27.90 b | 36.67 c |
| Phe | 2.47 a | 22.10 bc | 29.20 c | 59.07 e | 20.82 b | 20.50 b | 40.21 d |
| Lysine-hyd | 15.21 a | 42.65 b | 76.21 e | 90.56 f | 47.67 b | 61.68 c | 69.16 d |
| His | 55.51 a | 59.55 a | 77.58 c | 84.75 d | 57.91 a | 60.31 ab | 64.61 b |
| Arg | 18.10 a | 68.20 c | 81.77 d | 100.59 e | 64.57 bc | 64.74 bc | 59.58 b |
| Pro | 7.37 a | 25.17 b | 27.80 b | 46.39 d | 29.21 bc | 32.99 c | 52.80 e |
| Total | 264.29 a | 732.66 b | 959.96 f | 1272.10 g | 754.35 c | 792.81 d | 922.38 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Li, M.; Li, C.; Niu, M.; Dong, H.; Zhao, S.; Jia, C.; Xu, Y. Accelerated Accumulation of γ-Aminobutyric Acid and Modifications on Its Metabolic Pathways in Black Rice Grains by Germination under Cold Stress. Foods 2023, 12, 1290. https://doi.org/10.3390/foods12061290
Yu Y, Li M, Li C, Niu M, Dong H, Zhao S, Jia C, Xu Y. Accelerated Accumulation of γ-Aminobutyric Acid and Modifications on Its Metabolic Pathways in Black Rice Grains by Germination under Cold Stress. Foods. 2023; 12(6):1290. https://doi.org/10.3390/foods12061290
Chicago/Turabian StyleYu, Yingjie, Min Li, Chunxiao Li, Meng Niu, Huilong Dong, Siming Zhao, Caihua Jia, and Yan Xu. 2023. "Accelerated Accumulation of γ-Aminobutyric Acid and Modifications on Its Metabolic Pathways in Black Rice Grains by Germination under Cold Stress" Foods 12, no. 6: 1290. https://doi.org/10.3390/foods12061290