Royal Jelly and Chlorella vulgaris Mitigate Gibberellic Acid-Induced Cytogenotoxicity and Hepatotoxicity in Rats via Modulation of the PPARα/AP-1 Signaling Pathway and Suppression of Oxidative Stress and Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Animals
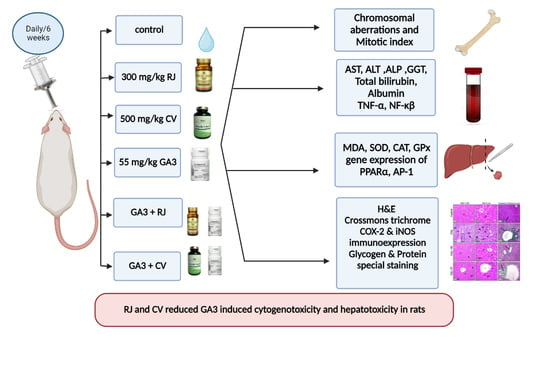
2.3. Experimental Plan and Sampling
2.4. Cytogenetic Assay
2.5. Estimation of Biochemical Assays
2.5.1. Assay of Liver Function Biomarkers
2.5.2. Assay of Oxidant/Antioxidant Indices
2.5.3. Assay of Serum TNF-α and NF-κB Levels
2.6. Quantitative Real Time-Polymerase Chain Reaction (qRT-PCR) for Detection of PPARα and AP-1 Genes Expression Level
2.7. Histopathological Assessment
2.7.1. Microscopic Evaluation
- a.
- General histological analysis:
- Haematoxylin and eosin (H&E) for general screening and semi-quantitative scoring of the hepatic damages in 6 fields X200 from each group. The hepatic alterations are cellular vacuolation, apoptosis, vascular congestion, and inflammation (inflammatory cell infiltrations). Scores were estimated as follows: 0 = no changes, 1 = alterations are <25%, 2 = alterations are 26–50%, 3 = alterations are 51–75%, and 4 = alterations are 75% of studied fields under the light microscope as measured by Gibson-Corley [43].
- Crossmon’s trichrome for clarification of collagen fibers and their area percentage.
- b.
- Histochemical analysis:
- Periodic acid-Schiff (PAS) stain for detection of cellular mucopolysaccharides secretions and their area percentage.
- Bromophenol blue stain for detection of cytoplasmic total protein contents and their area percentage.
- c.
- Immunohistochemical analysis: Applied on the paraffinized liver sections of all studied groups mounted on positive slides as follows:
- COX-2 immunohistochemistry: detection of COX-2 expression by using anti-COX-2 rabbit monoclonal antibody [EPR12012] (ab179800) [44]. The routine technique is performed till washing with water. Then, antigen retrieval was done by immersion of sections in Tris/EDTA buffer (10 mM Tris Base, 1 mM EDTA Solution, 0.05% Tween 20, pH 9) for 15 min, heat to 98 °C, and then let cool for 10–20 min. Sections must be flooded with 3% H2O2 for 5 min in methyl alcohol to suppress the endogenous peroxidase and then washed in TBS (10X, pH 7.4) plus 0.025% Triton X-100 with gentle agitation. At 37 °C, make blocking in 10% normal serum + 1% BSA in TBS for two hrs. Do not rinse and let slides dry. Applying the primary antibody (Anti-COX-2) diluted in TBS + 1% BSA 1:250 presented from Abcam, United States. Overnight incubation at 4 °C then rinse with gentle agitation in TBS 0.025% Triton. Apply the secondary antibody mingled with TBS + 1% BSA, and then incubate at 37 °C for 1 h. Stain sections with a chromogen Anti-COX-2 rabbit monoclonal antibody [EPR12012] (ab179800), at 37 °C for 10 min; then rinse with running tap water for 5 min. Stain the nuclei with Mayer’s hematoxylin for 2 min; then dehydrate, clear, and mount sections.
- iNOS immunohistochemistry: detection of iNOS expression by using Anti-iNOS rabbit polyclonal antibody (ab15323) [45]. In a water bath, make antigen retrieval by immersion of sections in sodium citrate buffer (10 mM Sodium Citrate, 0.05% Tween 20, pH 6.0) at 95 °C for 20 min, wash with PBS for 5 min, incubate sections with PBS (10X, pH 7.4) containing 0.1–0.25% Triton X-100 for 10 min, wash with PBS for 5 min, then stain with Anti-iNOS primary antibody presented from Abcam, Cambridge, UK, diluted 1:100 with 1% bovine serum albumin (BSA) + phosphate-buffered saline with Tween 20 (PBST) (1% BSA in PBST 1X, pH 7.0) at 4 °C for 12 h in a humidified chamber, rinse by TBS for 5 min, stain sections in the dark with the secondary antibody +1% BSA for 1 h at 37 °C, wash by PBS for 5 min in the dark, rinse with PBS, stain the nuclei with Mayer’s hematoxylin for 2 min, then dehydrate, clear, and mount sections.
2.7.2. Image Analysis
- Total collagen fibers in Crossmon’s trichrome-stained liver sections.
- Glycogen content in PAS-stained sections.
- Total protein content in Bromophenol blue-stained sections.
- Positive COX-2 content expression in immunostained sections with COX-2 antibody.
- Positive iNOS content expression in immunostained sections with iNOS antibody.
2.8. Statistical Analysis
3. Results
3.1. RJ and CV Decrease Cytogenetic Toxicity Induced by GA3 in Rats
| Groups | Structural Chromosomal Aberrations | Numerical Chromosomal Aberrations | TCA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chromatid Break | Chromatid Deletion | Ring Chromosome | Fragment | End to End Association | Centric Fusion | Centromeric Attenuation | TSA | Polyploidy | Aneuploidy | TNA | ||
| Control | 1 ± 1.3 | 0.7 ± 0.5 | 0.5 ± 0.8 | 0.5 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 2.7 ± 0.8 | 0.5 ± 0.5 | 0.3 ± 0.8 | 0.8 ± 1.2 | 3.5 ± 1 |
| RJ | 0.8 ± 1 | 0.5 ± 0.5 | 0.3 ± 0.4 | 0.3 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 2 ± 1 | 0.3 ± 0.5 | 0.2 ± 0.4 | 0.5 ± 0.5 | 2.5 ± 1 |
| CV | 1 ± 0. 9 | 0.7 ± 0.8 | 0.3 ± 0.5 | 0.5 ± 0.8 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 2.5 ± 1 | 0.5 ± 0.8 | 0.3 ± 0.5 | 0.8 ± 0.8 | 3.3 ± 0.8 |
| GA3 | 5.5 ± 1 *** | 6.7 ± 1 *** | 4.7 ± 1 *** | 2 ± 1.3 * | 1.5 ± 1*** | 1.7 ± 0.8 *** | 1.3 ± 1.2 ** | 23 ± 1 *** | 2 ± 1.3 * | 2.8 ± 1.5 *** | 4.8 ± 1.2 *** | 28 ± 0.8 *** |
| GA3 + RJ | 2.3 ± 1.2 ### | 2.7 ± 1.2 **### | 1.3 ± 1.2 ### | 0.5 ± 0.5 # | 0.3 ± 0.5 ## | 0.5 ± 0.8 ## | 0.2 ± 0.4 # | 7.8 ± 1.2 ***### | 0.5 ± 0.5 # | 0.8 ± 1 ## | 1.3 ± 0.8 ### | 9.2 ± 1.5 ***### |
| GA3 + CV | 2.7 ± 1.4 ## | 3.2 ± 1.2 ***### | 1.8 ± 1.5 ### | 0.7 ± 0.8 | 0.5 ± 0.5 # | 0.5 ± 0.5 ## | 0.3 ± 0.5 # | 9.7 ± 2 ***### | 0.7 ± 0.5 # | 1.2 ± 1 # | 1.8 ± 1 ### | 12 ± 2 ***### |
3.2. RJ and CV Ameliorate Liver Function Biomarker Changes Induced by GA3 in Rats
3.3. RJ and CV Alleviate Hepatic Oxidative Stress Induced by GA3 in Rats
3.4. RJ and CV Mitigate Inflammation Induced by GA3 in Rats
3.5. RJ and CV Up-Regulate PPAR Alpha and Down-Regulate AP-1 in the Liver of GA3-Intoxicated Rats
3.6. RJ and CV Attenuate Tissue Injury and Fibrosis in the Liver of GA3-Intoxicated Rats
| Groups | Vascular Congestion | Apoptosis | Cellular Vacuolation | Inflammation (Inflammatory Cell Infiltrations) |
|---|---|---|---|---|
| Control | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| RJ | 0.3 ± 0.6 | 0.3 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| CV | 0.7 ± 0.6 | 0.3 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| GA3 | 3.7 ± 0.6 *** | 3.3 ± 0.6 *** | 3.7 ± 0.6 *** | 3.7 ± 0.6 *** |
| GA3 + RJ | 0.7 ± 0.6 ### | 0.7 ± 0.6 ### | 0.7 ± 0.0 **### | 0.7 ± 0.6 ### |
| GA3 + CV | 1.3 ± 0.6 **### | 1.3 ± 0.6 **### | 1.3 ± 0.6 ***### | 1.3 ± 0.6 **### |
| Groups | Area Percentage of | ||||
|---|---|---|---|---|---|
| Collagen Fibers | PAS-Positive Content | Bromophenol Blue-Positive Content | COX-2 Immuno- Expression | iNOS Immuno- Expression | |
| Control | 1.3 ± 0.1 | 75.4 ± 1.4 | 77 ± 1 | 0.6 ± 0.1 | 0.13 ± 0.04 |
| RJ | 1.7 ± 0.2 | 74.3 ± 1.3 | 75 ± 1 | 0.6 ± 0.2 | 0.15 ± 0.03 |
| CV | 1.6 ± 0.3 | 73.4 ± 1.5 | 75 ± 1 | 0.7 ± 0.1 | 0.2 ± 0.1 |
| GA3 | 28 ± 2 *** | 23 ± 2 *** | 14 ± 1 *** | 70 ± 1.5 *** | 5.2 ± 1.6 *** |
| GA3 + RJ | 3.2 ± 0.5 **### | 71.5 ± 1.1 **### | 68.4 ± 1.1 ***### | 3.9 ± 0.7 **### | 0.7 ± 0.1 ### |
| GA3 + CV | 7.6 ± 0.8 ***### | 68 ± 2 ***### | 62 ± 2 ***### | 28.5 ± 2.4 ***### | 2.2 ± 0.6 **### |
3.7. RJ and CV Attenuate Histochemical Changes in the Liver of GA3-Intoxicated Rats
3.8. RJ and CV Downregulate COX2 and iNOS Immunoexpression in the Liver of GA3-Intoxicated Rats
3.9. Image Analysis and Statistical Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Végvári, G.; Vidéki, E. Plant hormones, plant growth regulators. Orv. Hetil. 2014, 155, 1011–1018. [Google Scholar] [CrossRef]
- Bao, S.; Hua, C.; Shen, L.; Yu, H. New insights into gibberellin signaling in regulating flowering in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 118–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwechheimer, C.; Willige, B.C. Shedding light on gibberellic acid signaling. Curr. Opin. Plant Biol. 2009, 12, 57–62. [Google Scholar] [CrossRef]
- Tomlin, C.D.S. Gibberellic acid. In The e-Pesticide Manual, 13th ed.; Tomlin, C.D.S., Ed.; British Crop Protection Council: Hampshire, UK, 2004; Chapters 3 and 5. [Google Scholar]
- Hussein, M.M.; Ali, H.A.; Ahmed, M.M. Ameliorative effects of phycocyanin against gibberellic acid induced hepatotoxicity. Pestic. Biochem. Physiol. 2015, 119, 28–32. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Protein oxidation. Ann. N. Y. Acad. Sci 2000, 899, 191–208. [Google Scholar] [CrossRef]
- Abdou, M.I.; Ayoub, M.A.; El Alem, M.M. Cytogenetic and pathological studies on the effect of gibberellic acid in rabbit. Egypt. J. Chem. Environ. Health 2016, 2, 566–579. [Google Scholar] [CrossRef]
- Sakr, S.A.; Sobhy, E.H.; Dalia, A.E. Effect of green tea on cytogenetic changes induced by gibberellin A3 in human lymphocyte culture. Can. J. Pure Appl. Sci. 2009, 3, 917–924. [Google Scholar]
- Abou-Eisha, A. Evaluation of cytogenetic and DNA damage induced by gibberellic acid. Toxicol. Vitr. 2001, 20, 601–607. [Google Scholar] [CrossRef]
- Alsemeh, A.E.; Moawad, R.S.; Abdelfattah, E.R. Histological and biochemical changes induced by gibberellic acid in the livers of pregnant albino rats and their offspring: Ameliorative effect of Nigella sativa. Anat. Sci. Int. 2019, 94, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Orfila, C.; Lepert, J.C.; Alric, L.; Carrera, G.; Béraud, M.; Pipy, B. Immunohistochemical distribution of activated nuclear factor κB and peroxisome proliferator-activated receptors in carbon tetrachloride-induced chronic liver injury in rats. Histochem. Cell Biol. 2005, 123, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Braissant, O.; Foufelle, F.; Scotto, C.; Dauca, M.; Wahli, W. Differential expression of peroxisome proliferator-activated receptors (PPARs): Tissue distribution of PPAR-a, -b, -c in the adult rat. Endocrinology 1996, 137, 354–366. [Google Scholar] [CrossRef] [Green Version]
- Staels, B.; Koenig, W.; Habib, A.; Merval, R.; Lebret, M.; Pineda-Torra, I.; Delerive, P.; Fadel, A.; Chinetti, G.; Fruchart, J.C.; et al. Activation of human aortic smooth-muscle cells is inhibited by PPARa but not by PPARg activators. Nature 1998, 393, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, S.; Kooshki, M.; Zhao, W.; Hsu, F.C.; Robbins, M.E. PPARalpha ligands inhibit radiation-induced microglial inflammatory responses by negatively regulating NF-kappaB and AP-1 pathways. Free Radic. Biol. Med. 2008, 15, 1695–1704. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.H.; Kim, S.; Choi, M.S.; Park, S.M.; Moon, K.S.; Yoon, S.; Oh, J.H. Inhibition of PPARα target genes during cyclosporine A-induced nephrotoxicity and hepatotoxicity. Mol. Cell. Toxicol. 2019, 15, 185–197. [Google Scholar] [CrossRef]
- Yang, X.N.; Liu, X.M.; Fang, J.H.; Zhu, X.; Yang, X.W.; Xiao, X.R.; Huang, J.F.; Gonzalez, F.J.; Li, F. PPARα Mediates the Hepatoprotective Effects of Nutmeg. J. Proteome Res. 2018, 17, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.M.; Khalaf, A.A.; Moselhy, W.A.; Safwat, G.M. Royal jelly attenuates azathioprine induced toxicity in rats. Environ. Toxicol. Pharmacol. 2014, 37, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Mokaya, H.O.; Njeru, L.K.; Lattorff, H.M.G. African honeybee royal jelly: Phytochemical contents, free radical scavenging activity, and physicochemical properties. Food Biosci. 2020, 37, 100733. [Google Scholar] [CrossRef]
- Hattori, N.; Nomoto, H.; Fukumitsu, H.; Mishima, S.; Furukawa, S. Royal jelly and its unique fatty acid, 10- hydroxy-trans-2-decenoic acid, promote neurogenesis by neural stem/ progenitor cell in vitro. Biomed. Res. 2007, 28, 261–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galaly, S.; Abdella, E.; Mohammed, H.; Khadrawy, S. Effects of royal jelly on genotoxicity and nephrotoxicity induced by valproic acid in albino mice. Beni-Suef Univ. J. Basic Appl. Sci. 2014, 3, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Tohamy, H.G.; El-Neweshy, M.S.; Soliman, M.M.; Sayed, S.; Shukry, M.; Ghamry, H.I.; Hoda, A.-E. Protective potential of royal jelly against hydroxyurea -induced hepatic injury in rats via antioxidant, anti-inflammatory, and anti-apoptosis properties. PLoS ONE 2022, 17, e0265261. [Google Scholar] [CrossRef]
- Zimmermann, A. Liver regeneration: The emergence of new pathways. Med. Sci. Monit. 2002, 8, RA53–RA63. [Google Scholar]
- Morris, H.J.; Almarales, A.; Carrill, O.; Bermudez, R.C. Utilisation of Chlorella vulgaris cell biomass for the production of enzymatic protein hydrolysates. Bioresour. Technol. 2008, 99, 7723–7729. [Google Scholar] [CrossRef]
- Bauer, L.M.; Vieira Costa, J.A.; Conteno da Rosa, A.P.; Santos, L.O. Growth stimulation and synthesis of lipids, pigments and antioxidants with magnetic fields in Chlorella kessleri cultivations. Bioresour. Technol. 2017, 244, 1425–1432. [Google Scholar] [CrossRef]
- Ajiboye, O.; Yakubu, A.; Adams, T. A perspective on the ingestion and nutritional effects of feed additives in farmed fish species. WJFMS 2012, 4, 87–101. [Google Scholar] [CrossRef]
- Kumar, M.; Jeon, J.; Choi, J.; Kim, S.-R. Rapid and efficient genetic transformation of the green microalga Chlorella vulgaris. J. Appl. Phycol. 2018, 30, 1735–1745. [Google Scholar] [CrossRef]
- Ko, S.C.; Kim, D.; Jeon, Y.J. Protective effect of a novel antioxidative peptide purified from a marine Chlorella ellipsoidea protein against free radical-induced oxidative stress. Food Chem. Toxicol. 2012, 50, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, R.E.; El-Marasy, S.A.; Abdel Jaleel, G.A.; Bakeer, R.M. Protective effect of royal jelly against diclofenac-induced hepato-renal damage and gastrointestinal ulcerations in rats. Heliyon 2020, 6, e03330. [Google Scholar] [CrossRef]
- Peng, H.Y.; Chu, Y.C.; Chen, S.J.; Chou, S.T. Hepatoprotection of Chlorella against carbon tetrachloride-induced oxidative damage in rats. In Vivo 2009, 23, 747–754. [Google Scholar] [PubMed]
- Soliman, M.M.; Aldhahrani, A.; Gaber, A.; Alsanie, W.F.; Shukry, M.; Mohamed, W.A.; Metwally, M.M.M. Impacts of n-acetyl cysteine on gibberellic acid-induced hepatorenal dysfunction through modulation of pro-inflammatory cytokines, antifibrotic and antioxidant activity. J. Food Biochem. 2021, 45, e13706. [Google Scholar] [CrossRef] [PubMed]
- Preston, R.; Dean, B.; Galloway, S.; Holden, H.; Mc-fee, A.; Shelby, M. Mammalian in vivo cytogenetic assays-analysis of chromosomal aberrations in bone marrow cells. Mutat. Res. 1987, 189, 157–165. [Google Scholar] [CrossRef]
- IFCC reference procedures for measurement of catalytic concentrations of enzymes: Corrigendum, notes and useful advice. Clin. Chem. Lab. Med. 2010, 48, 615–621.
- Burtis, C.A.; Ashwood, E.R.; Bruns, D.E. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 4th ed.; Elsevier Saunders: Paris, France, 2005. [Google Scholar]
- Young, D.S. Effects of Disease on Clinical Lab. Tests, 4th ed.; AACC: Washington, DC, USA, 2001. [Google Scholar]
- Doumas, B.T.; Biggs, H.G. Standard Methods of Clinical Chemistry; Academic Press: New York, NY, USA, 1976; Volume 7, p. 175. [Google Scholar]
- David, G.L.; Michael, D.L. Quantitative assessment of the multiple processes responsible for bilirubin homeostasis in health and disease. Clin. Exp. Gastroenterol. 2014, 7, 307–328. [Google Scholar] [CrossRef] [Green Version]
- Ohkawa, H.; Ohishi, W.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Nishikimi, M.; Roa, N.A.; Yogi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Bioph. Res. Common. 1972, 46, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013, 50, 1007–1015. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Ning, D.; Pan, J.; Chen, C.; Gao, C.; Ding, Z.; Jiang, F.; Li, M. Anti-Inflammatory Effect Fraction of Bletilla striata and Its Protective Effect on LPS-Induced Acute Lung Injury. Mediat. Inflamm. 2021, 13, 6684120. [Google Scholar] [CrossRef]
- Goh, B.J.; Tan, B.T.; Hon, W.M.; Lee, K.H.; Khoo, H.E. Nitric oxide synthase and heme oxygenase expressions in human liver cirrhosis. World J. Gastroenterol. 2006, 12, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Hussein, W.F.; Farahat, F.Y.; Abass, M.A.; Shehata, A.S. Hepatotoxic Potential of Ggibberellic Acid (GA3) in Adult Male Albino Rats. Life Sci. J. 2011, 8, 373–383. [Google Scholar]
- Seleem, A.A.; Hussein, B.H.M. Synthesis and effect of a new Terbium gibberellic complex on the histopathological alteration induced by Gibberellic acid on liver and kidney of mice Mus musculus. Chem. Biol. Drug Des. 2018, 92, 1288–1300. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Liu, C.; Luo, J.; Niu, C.; Wang, J.; Zheng, F.; Li, Q. Residue analysis of gibberellic acid isomer(iso-GA3) in brewing process and its toxicity evaluation in mice. Regul. Toxicol. Pharmacol. 2020, 110, 104514. [Google Scholar] [CrossRef] [PubMed]
- Zalinian, G.G.; Arutiunian, R.M.; Sarkisian, G.G. The cytogenetic effect of natural mutagenesis modifiers in a human lymphocyte culture. The action of aminobenzamide during the gibberellic acid induction of chromosome aberrations. Tsitol. Genet. 1990, 24, 31–34. [Google Scholar] [PubMed]
- Bakr, S.M.; Moussa, E.M.; Khater, E.S. Cytogenetic evaluation of gibberellin A3 in Swiss albino mice. J. Union Arab Biol. 1999, 11, 345–351. [Google Scholar]
- Nassar, S.A.; Fawzya, A.Z.; Ahmed, M.H.; Mohamed, N.M.; Asmaa, S.H. Cytogenetic, histological and histochemical studies on the effect of gibberllin A3 in albino rats. J. Am. Sci. 2012, 8, 608–622. [Google Scholar]
- Hassab-Elnabi, S.E.; Sallam, F.A. The protective effect of ellagic acid against the mutagenic potential of Berelex in human lymphocyte cultures. J. Egypt. Ger. Soc. Zool. 2002, 37, 77–98. [Google Scholar]
- Jovtchev, G.; Gateva, S.; Stergios, M.; Kulekova, S. Cytotoxic and genotoxic effects of paraquat in Hordeum vulgare and human lymphocytes in vitro. Environ. Toxicol. 2010, 25, 294–303. [Google Scholar] [CrossRef]
- El-Monem, D.A. The ameliorative effect of royal jelly against malathion genotoxicity in bone marrow and liver of rat. J. Am. Sci. 2011, 7, 1251–1256. [Google Scholar]
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New insights into the biological and pharmaceutical properties of royal jelly. Int. J. Mol. Sci. 2020, 21, 382. [Google Scholar] [CrossRef] [Green Version]
- EL Makawy, A.; Abdel-Aziem, S.; Ibrahim, F.; Sharaf, H.A.; Abd-Elmoneim, O.M.; Darwish, A. Potential modulator role of Chlorella vulgaris and Spirulina platensis on monosodium glutamate oxidative stress, genotoxicity, apoptotic gene expression and histopathological alterations. Int. J. Pharmtech. Res. 2016, 9, 161–177. [Google Scholar]
- Saberbaghi, T.; Abbasian, F.; Yusof, M.A.Y.; Makpol, S. Modulation of Cell Cycle Profile by Chlorella vulgaris Prevents Replicative Senescence of Human Diploid Fibroblasts. Evid. Based Complement. Alternat. Med. 2013, 2013, 780504. [Google Scholar] [CrossRef] [Green Version]
- Makpol, S.; Yaacob, N.; Zainuddin, A.; Yusof, M.A.Y.; Ngah, W.Z.W. Chlorella vulgaris modulates hydrogen peroxide-induced dna damage and telomere shortening of human fibroblasts derived from different aged individuals. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 560–572. [Google Scholar] [CrossRef] [Green Version]
- Tuluce, Y.; Celik, I. Influence of subacute and subchronic treatment of abcisic acid and gibberellic acid on serum marker enzymes and erythrocyte and tissue antioxidant defense systems and lipid peroxidation in rats. Pest. Biochem. Physiol. 2006, 86, 85–92. [Google Scholar] [CrossRef]
- Sakr, S.; Okdah, A.; Sabah, F.E.-A. Gibberellin A3 induced histological and histochemical alterations in the liver of albino rats. Sci. Asia 2003, 29, 327–331. [Google Scholar] [CrossRef]
- Jaeschke, H.; Gores, G.J.; Cederbaum, A.I.; Hinson, J.A.; Pessayre, D.; Lemasters, J.J. Mechanism of hepatoxicity. Toxicol. Sci. 2002, 56, 166–176. [Google Scholar] [CrossRef] [Green Version]
- Troudi, A.; Samet, A.M.; Zeghal, N. Hepatotoxicity induced by gibberellic acid in adult rats and their progeny. Exp. Toxicol. Pathol. 2010, 62, 637–642. [Google Scholar] [CrossRef]
- Farrugia, A. Albumin usage in clinical medicine: Tradition or therapeutic. Transfus. Med. Rev. 2010, 24, 53–63. [Google Scholar] [CrossRef]
- Nematalla, K.h.; Sahar, M.; Arafa, A.; Ghada, M.Y.; Zainb, A.S. Effect of Echinacea as Antioxidant on Markers of Aging. Aust. J. Basic Appl. Sci. 2011, 5, 18–26. [Google Scholar]
- Nkozi, C.Z.; Opoku, A.R.; Terblanche, S.E. Effect of pumpkin seed (Cucurbita pepo) protein isolate on the activity levels of certain plasma enzymes in CCl4-indued liver injury in low protein fed rats. Phytother. Res. 2005, 19, 341–345. [Google Scholar] [CrossRef]
- Fratini, F.; Cilia, G.; Mancini, S.; Felicioli, A. Royal Jelly: An ancient remedy with remarkable antibacterial properties. Microbiol. Res. 2016, 192, 130–141. [Google Scholar] [CrossRef]
- Gholie Pour, Z.; Nejati, V.; Najafi, G.; Pourzahra, K.; Khanshi, F. Protective effect of royal jelly on the adult female liver in experimental polycystic ovary syndrome. Qom Univ. Med. Sci. J. 2014, 8, 35–41. [Google Scholar]
- Kensler, T.W.; Egner, P.A.; Wang, J.B.; Zhu, Y.R.; Zhang, B.C.; Qian, G.S.; Kuang, S.Y.; Gange, S.J.; Jacobson, L.P.; Muñoz, A.; et al. Strategies for chemoprevention of liver cancer. Eur. J. Cancer Prev. 2002, 11, S58–S64. [Google Scholar] [PubMed]
- Vakili, J.; Amir Sasan, R.; Ordibazar, F. Effect of 8 weeks endurance training with Chlorella vulgaris supplementation on liver enzymes levels in women with type 2 diabetes. Med. J. Tabriz Univ. Med. Sci. 2019, 40, 88–97. [Google Scholar]
- Panahi, Y.; Ghamarchehreh, M.E.; Beiraghdar, F.; Zare, R.; Jalalian, H.R.; Sahebkar, A. Investigation of the effects of Chlorella vulgaris supplementation in patients with non-alcoholic fatty liver disease: A randomized clinical trial. Hepatogastroenterology 2012, 59, 2099–2103. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Mameghani, M.; Aliashrafi, S.; Javadzadeh, Y.; AsghariJafarabadi, M. The effect of Chlorella vulgaris supplementation on liver enzymes, serum glucose and lipid profile in patients with non-alcoholic fatty liver disease. Health Promot. Perspect. 2014, 4, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.Y.; Yang, L.Z. Liver Enzymes are Associated with Hyperglycemia in Diabetes: A Three-Year Retrospective Study. Diabetes Metab. Syndr. 2022, 15, 545–555. [Google Scholar] [CrossRef]
- Ou, Y.; Zheng, S.; Lin, L.; Jiang, Q.; Yang, X. Protective effect of C-phycocyanin against carbon tetrachloride-induced hepatocyte damage in vitro and in vivo. Chem. Biol. Interact. 2010, 185, 94–100. [Google Scholar] [CrossRef]
- Algeda, F.; Ebrahim, R. The Efficacy of Royal Jelly in the Restoration of Liver Injury in Irradiated Rats. EJRSA 2020, 33, 77–88. [Google Scholar] [CrossRef]
- You, M.M.; Liu, Y.C.; Chen, Y.F.; Pan, Y.M.; Miao, Z.N.; Shi, Y.Z.; Si, J.J.; Chen, M.L.; Hu, F.L. Royal jelly attenuates nonalcoholic fatty liver disease by inhibiting oxidative stress and regulating the expression of circadian genes in ovariectomized rats. J. Food Biochem. 2020, 44, e13138. [Google Scholar] [CrossRef]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant potential of propolis, bee pollen, and royal jelly: Possible medical application. Oxid. Med. Cell Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef]
- Tamura, S.; Kono, T.; Harada, C.; Yamaguchi, K.; Moriyama, T. Estimation and characterization of major royal jelly proteins obtained from the honeybee Apis merifera. Food Chem. 2009, 114, 1491–1497. [Google Scholar] [CrossRef]
- Khodabandeh, J.; Nejati, V.; Najafi, G.; Shalizar, J.; Ali, R.F. Protective effect of the royal gel on adipose tissue of adult females treated with nicotine. J. Neyshabur Sch. Med. Sci. 2017, 5, 22–31. [Google Scholar]
- Abdel-Tawwab, M.; Mousa, M.A.; Mamoon, A.; Abdelghany, M.F.; Abdel-Hamid, E.A.; Abdel-Razek, N.; Ali, F.S.; Shady, S.H.H.; Gewida, A.G.A. Dietary Chlorella vulgaris modulates the performance, antioxidant capacity, innate immunity, and disease resistance capability of Nile tilapia fingerlings fed on plant-based diets. Anim. Feed Sci. Technol. 2022, 283, 115181. [Google Scholar] [CrossRef]
- Coulombier, N.; Jauffrais, T.; Lebouvier, N. Antioxidant Compounds from Microalgae: A Review. Mar. Drugs 2021, 19, 549. [Google Scholar] [CrossRef]
- Zahran, E.; Risha, E. Modulatory role of dietary Chlorella vulgaris powder against arsenic-induced immunotoxicity and oxidative stress in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2014, 41, 654–662. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Yousefi, M.; Karimi, M.; Fadaei Raieni, R.; Dadar, M.; Yilmaz, S.; Dawood, M.A.O.; Abdel-Latif, H.M.R. Benefits of dietary polyphenols and polyphenol-rich additives to aquatic animal health: An overview. Rev. Fish. Sci. Aquac. 2021, 29, 478–511. [Google Scholar] [CrossRef]
- Shimada, M.; Hasegawa, T.; Nishimura, C.; Kan, H.; Kanno, T.; Nakamura, T.; Matsubayashi, T. Anti-hypertensive effect of γ-aminobutyric acid (GABA)-rich Chlorella on high-normal blood pressure and borderline hypertension in placebo controlled double-blind study. Clin. Exp. Hypertens. 2009, 31, 342–354. [Google Scholar] [CrossRef]
- Jadeja, R.N.; Devkar, R.V.; Nammi, S. Oxidative stress in liver diseases: Pathogenesis, prevention, and therapeutics. Oxid. Med. Cell Longev. 2017, 2017, 8341286. [Google Scholar] [CrossRef]
- Wilkinson-Berka, J.L.; Deliyanti, D.; Rana, I.; Miller, A.G.; Agrotis, A.; Armani, R.; Szyndralewiez, C.; Wingler, K.; Touyz, R.M.; Cooper, M.E.; et al. NADPH oxidase, NOX1, mediates vascular injury in ischemic retinopathy. Antioxid. Redox. Signal. 2014, 20, 2726–2740. [Google Scholar] [CrossRef]
- Barton, C.C.; Barton, E.X.; Ganey, P.E.; Kunkel, S.L.; Roth, R.A. Bacterial lipopoly saccharide enhance aflatoxin B1 hepatotoxicity in rats by a mechanism that depends on tumor necrosis factor alpha. Hepatology 2001, 33, 66–73. [Google Scholar] [CrossRef]
- Piao, R.L.; Liu, Y.Y.; Tian, D.; Ma, Z.H.; Zhang, M.; Zhao, C. Adefovir dipivoxil modulates cytokine expression in Th1/Th2 cells in patients with chronic hepatitis B. Mol. Med. Rep. 2012, 5, 184–189. [Google Scholar] [CrossRef]
- Estakhri, R.; Hajipour, B.; Majidi, H.; Soleimani, H. Vitamin E ameliorates cyclophosphamide induced nephrotoxicity. Life Sci. J. 2013, 10, 308–313. [Google Scholar]
- De Azevedo, M.T.; Saad, S.T.; Gilli, S.C. IL4 and IFN alpha generation of dendritic cells reveals great migratory potential and NF-kB and cJun expression in IL4DCs. Immunol. Investig. 2013, 42, 711–725. [Google Scholar] [CrossRef]
- Kim, M.E.; Jung, Y.C.; Jung, I.; Lee, H.W.; Youn, H.Y.; Lee, J.S. Anti-inflammatory effects of ethanolic extract from Sargassum horneri (Turner) C. Agardh on lipopolysaccharide-stimulated macrophage activation via NF-κB pathway regulation. Immunol. Investig. 2015, 44, 137–146. [Google Scholar] [CrossRef]
- Beg, A.A.; Baltimore, D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science 1996, 274, 782–784. [Google Scholar] [CrossRef]
- Almeer, R.S.; Alarifi, S.; Alkahtan, S.; Ibrahim, S.R.; Ali, D.; Abde Moneim, H. The potential hepatoprotective effect of royal jelly against cadmium chloride-induced hepatotoxicity in mice is mediated by suppression of oxidative stress and upregulation of Nrf2 expression. Biomed. Pharmacother. 2018, 106, 1490–1498. [Google Scholar] [CrossRef]
- Abu-Serie, M.M.; Habashy, N.H.; Attia, W.E. In vitro evaluation of the synergistic antioxidant and anti-inflammatory activities of the combined extracts from Malaysian Ganoderma lucidum and Egyptian Chlorella vulgaris. BMC Complement. Altern. Med. 2018, 18, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, M.T.; Almeida, J.R.; Araujo, A.A.; Duarte, M.C.; Gelain, D.P.; Moreira, J.C.; Dos Santos, M.R.; Quintans-Junior, L.J. Structure-activity relationship of terpenes with anti-inflammatory profile–asystematic review. Basic Clin. Pharmacol. Toxicol. 2014, 115, 244–256. [Google Scholar] [CrossRef]
- Caroprese, M.; Albenzio, M.; Ciliberti, M.G.; Francavilla, M.; Sevi, A. A mixture of phytosterols from Dunaliella tertiolecta affects proliferation of peripheral blood mononuclear cells and cytokine production in sheep. Vet. Immunol. Immunopathol. 2012, 150, 27–35. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, W.; Gan, L.; Liu, S.; Ni, Q.; Bi, Y.; Han, T.; Liu, Q.; Chen, H.; Hu, Y.; et al. Silencing HIF-1α aggravates non-alcoholic fatty liver disease in vitro through inhibiting PPAR-α/ANGPTL4 singling pathway. Gastroenterol. Hepatol. 2021, 44, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Gervois, P.; Vu-Dac, N.; Kleemann, R.; Kockx, M.; Dubois, G.; Laine, B.; Kosykh, V.; Fruchart, J.C.; Kooistra, T.; Staels, B. Negative regulation of human fibrinogen gene expression by peroxisome proliferator-activated receptor alpha agonists via inhibition of CCAAT box/enhancer-binding protein beta. J. Biol. Chem. 2001, 276, 33471–33477. [Google Scholar] [CrossRef] [Green Version]
- Pawlak, M.; Baugé, E.; Bourguet, W.; De Bosscher, K.; Lalloyer, F.; Tailleux, A.; Lebherz, C.; Lefebvre, P.; Staels, B. The transrepressive activity of peroxisome proliferator-activated receptor alpha is necessary and sufficient to prevent liver fibrosis in mice. Hepatology 2014, 60, 1593–1606. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, H.M.; Kaestner, K.H.; Tuckermann, J.; Kretz, O.; Wessely, O.; Bock, R.; Gass, P.; Schmid, W.; Herrlic, P.; Angle, P.; et al. DNA binding of the glucocorticoid receptor is not essential for survival. Cell 1998, 93, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, M.; Hayashi, K.; Watadani, R.; Okano, Y.; Tanimura, K.; Kotoh, J.; Sasaki, D.; Matsumoto, K.; Maeda, A. Royal jelly improves hyperglycemia in obese/diabetic KK-Ay mice. J. Vet. Med. Sci. 2017, 79, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, S.S.; Halbower, A.; Pan, Z.; Robbins, K.; Capocelli, K.E.; Klawitter, J.; Shearn, C.T.; Sokol, R.J. Nocturnal hypoxia-induced oxidative stress promotes progression of pediatric non-alcoholic fatty liver disease. J. Hepatol. 2016, 65, 560–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dembinska-Kiec, A. Carotenoids: Risk or benefit for health’, Carotenoids Diet. Lipids 2005, 1740, 93–94. [Google Scholar]
- Ali, S.; Moselhy, W.; Mohamed, H.; Nabil, T.; Abo El-Ela, F.; Abdou, K. Ameliorative effects of Dictyota dichotoma on hepatotoxicity induced by gibberellic acid in albino rats. Toxicol. Res. 2022, 38, 379–392. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Wang, C.X. Histopathological and histochemical studies on the toxic effect of brodifacoum in mouse liver. Acta Acad. Med. Sci. 1984, 6, 386–388. [Google Scholar]
- Izunya, A.M.; Nwaopara, A.O.; Odike, M.A.C.; Oaikhena, G.A.; Bankole, J.K. Histological effects of oral administration of artesunate on the liver in Wistar rats. Res. J. Appl. Sci. Eng. Technol. 2010, 2, 314–318. [Google Scholar]
- Rahman, I.; MacNee, W. Oxidative stress and regulation of glutathione in lung inflammation. Eur. Respir. J. 2000, 16, 534–554. [Google Scholar] [CrossRef]
- Cemek, M.; Aymelek, F.; Büyükokuroğlu, M.E.; Karaca, T.; Büyükben, A.; Yilmaz, F. Protective potential of Royal Jelly against carbon tetrachloride-induced toxicity and changes in the serum sialic acid levels. Food Chem. Toxicol. 2010, 48, 2827–2832. [Google Scholar] [CrossRef]
- Naguib, Y.M. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef]
- El-Fayoumy, E.A.; Shanab, S.M.M.; Gaballa, H.S.; Tantawy, M.A.; Shalaby, E.A. Evaluation of antioxidant and anticancer activity of crude extract and different fractions of Chlorella vulgaris axenic culture grown under various concentrations of copper ions. BMC Complement. Med. Ther. 2021, 21, 51. [Google Scholar] [CrossRef]
- Alshawsh, M.A.; Abdulla, M.A.; Ismail, S.; Amin, Z.A. Hepatoprotective effects of Orthosiphon stamineus extract on thioacetamide-induced liver cirrhosis in rats. Evid. Based Complement. Alternat. Med. 2011, 2011, 103039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, M.; Schuppan, D. TGF beta1 in liver fibrosis: Time to change paradigms? FEBS Lett. 2001, 502, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Ross, M.; Pawlina, W. “Histology”: A Text and Atlas with Correlated Cell and Molecular Biology, 5th ed.; Lippincott: New York, NY, USA; Williams & Wilkins: Philadelphia, PA, USA, 2006; pp. 576–584. [Google Scholar]
- Aslan, A.; Gok, O.; Beyaz, S.; Can, M.I.; Parlak, G.; Gundogdu, R.; Ozercan, I.H.; Baspinar, S. Royal jelly regulates the caspase, Bax, and COX-2, TNF-α protein pathways in the fluoride-exposed lung damage in rats. Tissue Cell 2022, 76, 101754. [Google Scholar] [CrossRef]
- Denda, A.; Kitayama, W.; Murata, A.; Kishida, H.; Sasaki, Y.; Kusuoka, O.; Tsujiuchi, M.; Tsutsumi, M.; Nakae, D.; Takagi, H.; et al. Increased expression of cyclooxygenase-2 protein during rat hepatocarcinogenesis caused by a choline-deficient, L-amino acid-defined diet and chemopreventive efficacy of a specific inhibitor, nimesulfide. Carcinogenesis 2002, 23, 245–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, F.C.; Feng, J.J.; Chen, K.H.; Imanishi, H.; Fujishima, M.; Takekoshi, H.; Naoki, Y.; Shimoda, M. Chlorella powder inhibits the activities of peptidase cathepsin S, PLA2, cyclooxygenase-2, thromboxane synthase, tyrosine phosphatases, tumor necrosis factor-alpha converting enzyme, calpain, and kinases. Int. J. Food Sci. Nutr. 2009, 60, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.A.; Abd El-Aleem, S.; Appleton, I.; Maklouf, M.M.; Said, M.; McMahon, R.F. Expression of nitric oxide synthase isoforms in human liver cirrhosis. J. Pathol. 2003, 200, 647–655. [Google Scholar] [CrossRef]
- Tache, D.; Stănciulescu, C.; Baniţă, I.; Purcaru, Ş.; Andrei, A.; Comănescu, V.; Pisoschi, C. Inducible nitric oxide synthase expression (iNOS) in chronic viral hepatitis and its correlation with liver fibrosis. Rom. J. Morphol. Embryol. 2014, 55, 539–543. [Google Scholar] [PubMed]
- Karaca, T.; Bayiroglu, F.; Yoruk, M.; Kaya, M.S.; Uslu, S.; Comba, B.; Mis, L. Effect of royal jelly on experimental colitis Induced by acetic acid and alteration of mast cell distribution in the colon of rats. Eur. J. Histochem. 2012, 21, e35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Cho, H.; Kim, J.; Noh, K.; Yang, J.; Ahn, J.; Lee, M.; Song, Y. Chlorella dichloromethane extract ameliorates NO production and iNOS expression through the down-regulation of NFκB activity mediated by suppressed oxidative stress in RAW 264.7 macrophages. Clin. Chim. Acta 2005, 351, 185–196. [Google Scholar] [CrossRef] [PubMed]






| Gene | Sequence 5′–3′ | Gene Accession Number |
|---|---|---|
| PPARα | Forward sequence: TTC GGA AAC TGC AGA CCT Reverse sequence: TTA GGA ACT CTC GGG TGA T | NC_051342.1 |
| AP-1 | Forward sequence: CAA CGC CTC GTT CCT CCC G Reverse sequence: GGC GCG GAG GTG CGG CTT C | NC_051341.1 |
| β-actin | Forward sequence: AGG TCA TCA CTA TCG GCA AT Reverse sequence: ACT CAT CGT ACT CCT GCT TG | NC_051347.1 |
| Groups | ALT (U/L) | AST (U/L) | ALP (U/L) | γGT (U/L) | Albumin (g/dL) | Total Bilirubin (mg/dL) |
|---|---|---|---|---|---|---|
| Control | 75 ± 2 | 113 ± 13 | 219 ± 8 | 14 ± 1 | 4.3 ± 0.4 | 0.8 ± 0.1 |
| RJ | 69 ± 3 | 110 ± 6 | 214 ± 12 | 13 ± 1 | 4.7 ± 0.3 | 0.7 ± 0.1 |
| CV | 75 ± 5 | 113 ± 5 | 216 ± 12 | 13 ± 2 | 4.3 ± 0.4 | 0.75 ± 0.04 |
| GA3 | 139 ± 11 *** | 199 ± 7 *** | 479 ± 16 *** | 25 ± 2 *** | 1.9 ± 0.1 *** | 1.5 ± 0.1 *** |
| GA3 + RJ | 85 ± 8 ### | 129 ± 9 ### | 378 ± 19 ***### | 15 ± 1 ### | 3.8 ± 0.2 ### | 0.9 ± 0.1 ### |
| GA3 + CV | 96 ± 3 *### | 148 ± 2 **### | 413 ± 5 ***### | 17 ± 0.5 ### | 3.3 ± 0.5 *## | 1 ± 0.1 *### |
| Groups | MDA (nmol/g) | SOD (U/g) | CAT (U/L) | GPx (U/g) |
|---|---|---|---|---|
| Control | 1.3 ± 0.2 | 2.1 ± 0.5 | 2.9 ± 0.2 | 2 ± 0.1 |
| RJ | 1.1 ± 0.1 | 2.2 ± 0.2 | 2.9 ± 0.2 | 2.3 ± 0.2 |
| CV | 1.3 ± 0.3 | 2.2 ± 0.1 | 2.9 ± 0.2 | 2 ± 0.1 |
| GA3 | 2.6 ± 0.2 *** | 0.72 ± 0.03 *** | 0.8 ± 0.02 *** | 0.8 ± 0.1 *** |
| GA3 + RJ | 1.6 ± 0.1 ### | 1.82 ± 0.04 ### | 2 ± 0.1 ***### | 1.7 ± 0.2 ### |
| GA3 + CV | 1.8 ± 0.1 *### | 1.77 ± 0.04 ### | 2 ± 0.2 ***### | 1.6 ± 0.2 *### |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khadrawy, S.M.; Mohamed, D.S.; Hassan, R.M.; Abdelgawad, M.A.; Ghoneim, M.M.; Alshehri, S.; Shaban, N.S. Royal Jelly and Chlorella vulgaris Mitigate Gibberellic Acid-Induced Cytogenotoxicity and Hepatotoxicity in Rats via Modulation of the PPARα/AP-1 Signaling Pathway and Suppression of Oxidative Stress and Inflammation. Foods 2023, 12, 1223. https://doi.org/10.3390/foods12061223
Khadrawy SM, Mohamed DS, Hassan RM, Abdelgawad MA, Ghoneim MM, Alshehri S, Shaban NS. Royal Jelly and Chlorella vulgaris Mitigate Gibberellic Acid-Induced Cytogenotoxicity and Hepatotoxicity in Rats via Modulation of the PPARα/AP-1 Signaling Pathway and Suppression of Oxidative Stress and Inflammation. Foods. 2023; 12(6):1223. https://doi.org/10.3390/foods12061223
Chicago/Turabian StyleKhadrawy, Sally M., Doaa Sh. Mohamed, Randa M. Hassan, Mohamed A. Abdelgawad, Mohammed M. Ghoneim, Sultan Alshehri, and Nema S. Shaban. 2023. "Royal Jelly and Chlorella vulgaris Mitigate Gibberellic Acid-Induced Cytogenotoxicity and Hepatotoxicity in Rats via Modulation of the PPARα/AP-1 Signaling Pathway and Suppression of Oxidative Stress and Inflammation" Foods 12, no. 6: 1223. https://doi.org/10.3390/foods12061223