Potato Chips Byproducts as Feedstocks for Developing Active Starch-Based Films with Potential for Cheese Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
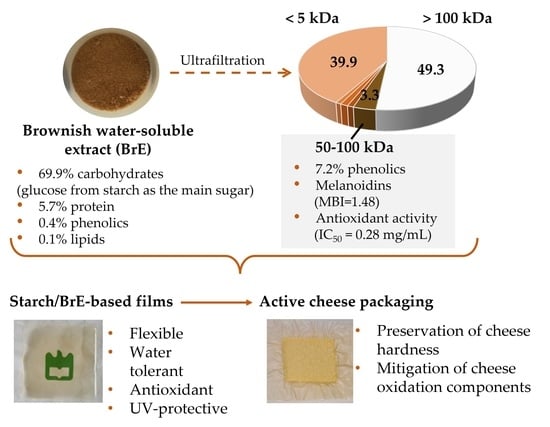
2.2. Fractionation of Potato Chip Brownish Water-Soluble Extract (BrE)
2.3. Characterization of Potato Chip Brownish Water-Soluble Extract (BrE)
2.4. Production of Starch/BrE-Based Films by Solvent Casting
2.5. Characterization of the Starch/BrE-Based Films
2.5.1. Chromatic Properties
2.5.2. Thickness and Mechanical Properties
2.5.3. Wettability
2.5.4. Water Solubility
2.5.5. Water Vapor Transmission Rate
2.5.6. UV-Protective and Antioxidant Activity
2.6. Sliced Cheese Package with Starch/15% BrE-Based Films
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Brownish Water-Soluble Extract (BrE)
3.2. Characterization of the Potato Starch/BrE-Based Films
3.2.1. Chromatic Properties
3.2.2. Thickness and Mechanical Properties
3.2.3. Wettability
3.2.4. Solubility and Water Vapor Permeability
3.2.5. UV-Protective and Antioxidant Properties
3.3. Effect of Potato Starch/BrE-Based Films on Packed Sliced Cheese Quality
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT Statistics. Potatoes Production in the World. 2020. Available online: http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567#ancor (accessed on 19 November 2022).
- Pedreschi, F. Frying of Potatoes: Physical, Chemical, and Microstructural Changes. Dry. Technol. 2012, 30, 707–725. [Google Scholar] [CrossRef]
- Basuny, A.M.M.; Mostafa, D.M.M.; Shaker, A.M. Relationship Between Chemical Composition and Sensory Evaluation of Potato Chips Made from Six Potato Varieties with Emphasis on the Quality of Fried Sunflower Oil. World J. Dairy Food Sci. 2009, 4, 193–200. [Google Scholar]
- Freitas, L.; Barbosa, J.; da Costa, A.; Bezerra, F.; Pinto, R.; de Carvalho, R. From waste to sustainable industry: How can agro-industrial wastes help in the development of new products? Resour. Conserv. Recy. 2021, 169, 105466. [Google Scholar] [CrossRef]
- Goncalves, I.; Lopes, J.; Barra, A.; Hernandez, D.; Nunes, C.; Kapusniak, K.; Kapusniak, J.; Evtyugin, D.; da Silva, J.; Ferreira, P.; et al. Tailoring the surface properties and flexibility of starch-based films using oil and waxes recovered from potato chips byproducts. Int. J. Biol. Macromol. 2020, 163, 251–259. [Google Scholar] [CrossRef]
- Petronilho, S.; Oliveira, A.; Domingues, M.R.; Nunes, F.M.; Coimbra, M.A.; Gonçalves, I. Hydrophobic Starch-Based Films Using Potato Washing Slurries and Spent Frying Oil. Foods 2021, 10, 2897. [Google Scholar] [CrossRef]
- Lopes, J.; Gonçalves, I.; Nunes, C.; Teixeira, B.; Mendes, R.; Ferreira, P.; Coimbra, M.A. Potato peel phenolics as additives for developing active starch-based films with potential to pack smoked fish fillets. Food Pack. Shelf Life 2021, 28, 100644. [Google Scholar] [CrossRef]
- Hosseinzadeh-Bandbafha, H.; Nizami, A.; Kalogirou, S.; Gupta, V.; Park, Y.; Fallahi, A.; Sulaiman, A.; Ranjbari, M.; Rahnama, H.; Aghbashlo, M.; et al. Environmental life cycle assessment of biodiesel production from waste cooking oil: A systematic review. Renew. Sust. Energy Rev. 2022, 161, 112411. [Google Scholar] [CrossRef]
- Mesias, M.; Delgado-Andrade, C. Melanoidins as a potential functional food ingredient. Curr. Opin. Food Sci. 2017, 14, 37–42. [Google Scholar] [CrossRef]
- Moreira, A.; Coimbra, M.; Nunes, F.; Passos, C.; Santos, S.; Silvestre, A.; Silva, A.; Rangel, M.; Domingues, M. Chlorogenic acid-arabinose hybrid domains in coffee melanoidins: Evidences from a model system. Food Chem. 2015, 185, 135–144. [Google Scholar] [CrossRef]
- Moreira, A.; Nunes, F.; Domingues, M.; Coimbra, M. Coffee melanoidins: Structures, mechanisms of formation and potential health impacts. Food Funct. 2012, 3, 903–915. [Google Scholar] [CrossRef]
- Petronilho, S.; Navega, J.; Pereira, C.; Almeida, A.; Siopa, J.; Nunes, F.M.; Coimbra, M.A.; Passos, C.P. Bioactive Properties of Instant Chicory Melanoidins and Their Relevance as Health Promoting Food Ingredients. Foods 2023, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Passos, C.; Costa, R.; Ferreira, S.; Lopes, G.; Cruz, M.; Coimbra, M. Role of Coffee Caffeine and Chlorogenic Acids Adsorption to Polysaccharides with Impact on Brew Immunomodulation Effects. Foods 2021, 10, 378. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.R.; Passos, C.P.; Petronilho, S.; Rodrigues, C.; Teixeira, J.A.; Coimbra, M.A. Carbohydrates as targeting compounds to produce infusions resembling espresso coffee brews using quality by design approach. Food Chem. 2021, 344, 128613. [Google Scholar] [CrossRef] [PubMed]
- Vangelder, W. Conversion factor from nitrogen to protein for potato-tuber protein. Potato Res. 1981, 24, 423–425. [Google Scholar] [CrossRef]
- Bekedam, E.; Schols, H.; Van Boekel, M.; Smit, G. High molecular weight melanoidins from coffee brew. J. Agric. Food Chem. 2006, 54, 7658–7666. [Google Scholar] [CrossRef]
- Passos, C.; Cepeda, M.; Ferreira, S.; Nunes, F.; Evtuguin, D.; Madureira, P.; Vilanova, M.; Coimbra, M. Influence of molecular weight on in vitro immunostimulatory properties of instant coffee. Food Chem. 2014, 161, 60–66. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Oliveira, G.; Goncalves, I.; Barra, A.; Nunes, C.; Ferreira, P.; Coimbra, M. Coffee silverskin and starch-rich potato washing slurries as raw materials for elastic, antioxidant, and UV-protective biobased films. Food Res. Int. 2020, 138, 109733. [Google Scholar] [CrossRef]
- Benedito, J.; Simal, S.; Clemente, G.; Mulet, A. Manchego cheese texture evaluation by ultrasonics and surface probes. Int. Dairy J. 2006, 16, 431–438. [Google Scholar] [CrossRef]
- Sarfraz, J.; Hansen, A.; Haugen, J.; Le, T.; Nilsen, J.; Skaret, J.; Huynh, T.; Pettersen, M. Biodegradable Active Packaging as an Alternative to Conventional Packaging: A Case Study with Chicken Fillets. Foods 2021, 10, 1126. [Google Scholar] [CrossRef]
- Coimbra, M.; Waldron, K.; Delgadillo, I.; Selvendran, R. Effect of processing on cell wall polysaccharides of green table olives. J. Agric. Food Chem. 1996, 44, 2394–2401. [Google Scholar] [CrossRef]
- Subramanian, N.; White, P.; Broadley, M.; Ramsay, G. The three-dimensional distribution of minerals in potato tubers. Ann. Bot. 2011, 107, 681–691. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Galvez, A.; Castro-Rosas, J.; Rodriguez-Marin, M.; Cadena-Ramirez, A.; Tellez-Jurado, A.; Tovar-Jimenez, X.; Chavez-Urbiola, E.; Abreu-Corona, A.; Gomez-Aldapa, C. Antimicrobial activity and physicochemical characterization of a potato starch-based film containing acetonic and methanolic extracts of Hibiscus sabdariffa for use in sausage. LWT-Food Sci. Technol. 2018, 93, 300–305. [Google Scholar] [CrossRef]
- Goncalves, I.; Hernandez, D.; Cruz, C.; Lopes, J.; Barra, A.; Nunes, C.; da Silva, J.; Ferreira, P.; Coimbra, M. Relevance of genipin networking on rheological, physical, and mechanical properties of starch-based formulations. Carbohydr. Polym. 2021, 254, 117236. [Google Scholar] [CrossRef]
- Luchese, C.; Garrido, T.; Spada, J.; Tessaro, I.; de la Caba, K. Development and characterization of cassava starch films incorporated with blueberry pomace. Int. J. Biol. Macromol. 2018, 106, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Albishi, T.; John, J.; Al-Khalifa, A.; Shahidi, F. Phenolic content and antioxidant activities of selected potato varieties and their processing by-products. J. Funct. Foods 2013, 5, 590–600. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Holm, V.; Mortensen, G.; Risbo, J. Quality changes in semi-hard cheese packaged in a poly(lactic acid) material. Food Chem. 2006, 97, 401–410. [Google Scholar] [CrossRef]
- Petronilho, S.; Lopez, R.; Ferreira, V.; Coimbra, M.A.; Rocha, S.M. Revealing the Usefulness of Aroma Networks to Explain Wine Aroma Properties: A Case Study of Portuguese Wines. Molecules 2020, 25, 272–289. [Google Scholar] [CrossRef] [Green Version]
- Bagler, G. FlavorDB Search: A Resource for Exploring Flavor Molecules. Center for Computational Biology, Indraprastha Institute of Information Technology Delhi (IIIT Delhi), Academic Block, 2017. Available online: http://cosylab.iiitd.edu.in/flavordb (accessed on 6 December 2022).









| Sample | Yield * | Ara | Gal | Glc | Total Sugars | Total Protein | Total Phenolics | Total EFA | Kmix,405 nm | MBI | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % (w/w) | mol% | % (w/w) | (L/g/cm) | |||||||||||
| BrE | 57.2 | 0.95 | 5.20 | 93.9 | 66.9 | 5.70 | 0.40 | 0.10 | n.d. | n.d. | ||||
| >100 kDa | 49.3 | 1.19 | 6.06 | 92.7 | 89.4 | 7.60 | 0.90 | 0.11 | 0.27 | n.d. | ||||
| 50–100 kDa | 3.30 | 2.62 | 12.02 | 85.4 | 29.4 | 17.4 | 7.20 | 0.16 | 0.69 | 1.48 | ||||
| <5 kDa | 39.9 | 4.50 | 12.43 | 83.1 | 37.1 | 16.4 | 1.10 | 0.10 | 0.14 | 0.31 | ||||
| Sliced Cheese Samples | ||||||
|---|---|---|---|---|---|---|
| Weight Loss | Chromatic Parameters | |||||
| Image | Time (Days) | (%) | L* | a* | b* | ΔE |
| PA/PE-based materials | ||||||
 | 0 | - | 81.44 ± 0.57 a | −2.86 ± 0.18 a | 31.04 ± 0.53 a | - |
 | 7 | 0.12 ± 0.04 a | 79.68 ± 0.50 b | −3.57 ± 0.04 b | 30.17 ± 0.31 a,b | 2.09 |
 | 14 | 0.02 ± 0.03 a | 81.74 ± 0.91 a,b | −2.44 ± 0.13 c | 31.12 ± 0.49 a | 0.52 |
| Starch/15% BrE-based materials | ||||||
 | 0 | - | 81.44 ± 0.57 a | −2.86 ± 0.18 a | 31.04 ± 0.53 a | - |
 | 7 | 6.52 ± 2.16 b | 76.10 ± 2.01 c | −2.96 ± 0.21 a | 30.84 ± 0.49 a | 5.34 |
 | 14 | 6.54 ± 1.58 b | 77.12 ± 1.99 b,c | −1.81 ± 0.19 d | 34.17 ± 1.59 c | 5.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peixoto, A.M.; Petronilho, S.; Domingues, M.R.; Nunes, F.M.; Lopes, J.; Pettersen, M.K.; Grøvlen, M.S.; Wetterhus, E.M.; Gonçalves, I.; Coimbra, M.A. Potato Chips Byproducts as Feedstocks for Developing Active Starch-Based Films with Potential for Cheese Packaging. Foods 2023, 12, 1167. https://doi.org/10.3390/foods12061167
Peixoto AM, Petronilho S, Domingues MR, Nunes FM, Lopes J, Pettersen MK, Grøvlen MS, Wetterhus EM, Gonçalves I, Coimbra MA. Potato Chips Byproducts as Feedstocks for Developing Active Starch-Based Films with Potential for Cheese Packaging. Foods. 2023; 12(6):1167. https://doi.org/10.3390/foods12061167
Chicago/Turabian StylePeixoto, Ana M., Sílvia Petronilho, M. Rosário Domingues, Fernando M. Nunes, Joana Lopes, Marit Kvalvåg Pettersen, Magnhild S. Grøvlen, Elin M. Wetterhus, Idalina Gonçalves, and Manuel A. Coimbra. 2023. "Potato Chips Byproducts as Feedstocks for Developing Active Starch-Based Films with Potential for Cheese Packaging" Foods 12, no. 6: 1167. https://doi.org/10.3390/foods12061167