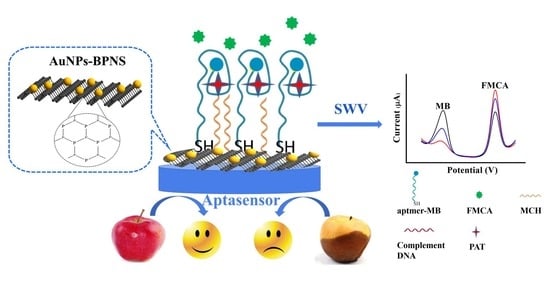
A Dual-Signaling Electrochemical Aptasensor Based on an In-Plane Gold Nanoparticles–Black Phosphorus Heterostructure for the Sensitive Detection of Patulin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus and Characterization
2.3. Preparation of BPNS
2.4. Preparation of AuNPs–BPNS
2.5. Preparation of the Modified Electrodes
2.6. Electrochemical Detection of PAT
2.7. Pretreatment of Food Samples
3. Results
3.1. Characterization of the AuNPs–BPNS
3.2. Characterization of the Modified Electrode
3.3. Electrochemical PAT Assay
3.4. Reproducibility, Stability and Selectivity
3.5. Real Sample Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bennett, J.; Klich, M. Clinical Microbiology Reviews. Mycotoxins 2003, 16, 497. [Google Scholar]
- Marín, S.; Mateo, E.M.; Sanchis, V.; Valle-Algarra, F.M.; Ramos, A.J.; Jiménez, M. Patulin contamination in fruit derivatives, including baby food, from the Spanish market. Food Chem. 2011, 124, 563–568. [Google Scholar] [CrossRef]
- Leggott, N.L.; Shephard, G.S. Patulin in South African commercial apple products. Food Control. 2001, 12, 73–76. [Google Scholar] [CrossRef]
- Wu, N.; Wang, Y.; Liu, Y.; Song, J.; Yang, X.; Su, C.; Liu, X. Investigation on the content of penicillin in fruit products in some areas of China. Health Res. 1992, 2, 101–103. [Google Scholar]
- Veprikova, Z.; Vaclavikova, M.; Lacina, O.; Dzuman, Z.; Zachariasova, M.; Hajslova, J. Occurrence of mono- and di-glycosylated conjugates of T-2 and HT-2 toxins in naturally contaminated cereals. World Mycotoxin J. 2012, 5, 231–240. [Google Scholar] [CrossRef]
- Seo, M.; Kim, B.; Baek, S.-Y. An optimized method for the accurate determination of patulin in apple products by isotope dilution-liquid chromatography/mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 5433–5442. [Google Scholar] [CrossRef]
- Yang, J.Z.; Wei, W.; Zhu, W.X.; Liu, Y.F. Extraction and preliminary purification of cochineal wax. Food Sci. 2009, 30, 162–165. [Google Scholar]
- Kharandi, N.; Babri, M.; Azad, J. A novel method for determination of patulin in apple juices by gc-ms. Food Chem. 2013, 141, 1619–1623. [Google Scholar] [CrossRef]
- Lu, C.; Chen, X.; Liu, C.; Lu, C. Development and validation of a label-free colorimetric aptasensor based on the HCR and hemin/G-quadruplex DNAzyme for the determination of patulin in fruits and fruit-based products from Xinjiang (China). Anal. Methods 2022, 14, 3375–3381. [Google Scholar] [CrossRef]
- Pennacchio, A.; Varriale, A.; Esposito, M.G.; Staiano, M.; D’Auria, S. A near-infrared fluorescence assay method to detect patulin in food. Anal. Biochem. 2015, 481, 55–59. [Google Scholar] [CrossRef]
- Pennacchio, A.; Ruggiero, G.; Staiano, M.; Piccialli, G.; Oliviero, G.; Lewkowicz, A.; Synak, A.; Bojarski, P.; D’Auria, S. A surface plasmon resonance based biochip for the detection of patulin toxin. Opt. Mater. 2014, 36, 1670–1675. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.; Luo, Y.; Yang, X.; Li, M.; Song, Q. Double Detection of Mycotoxins Based on SERS Labels Embedded Ag@Au Core–Shell Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 21780–21786. [Google Scholar] [CrossRef]
- Esteban-Fernández de Ávila, B.; Lopez-Ramirez, M.A.; Ba, D.F.; Jodra, A.; Singh, V.V.; Kaufmann, K.; Wang, J. Aptamer-modified graphene-based catalytic micromotors: Off–on fluorescent detection of ricin. ACS Sens. 2016, 1, 217–221. [Google Scholar] [CrossRef]
- Cui, M.; Wang, Y.; Jiao, M.; Jayachandran, S.; Wu, Y.; Fan, X.; Luo, X. Mixed Self-Assembled Aptamer and Newly Designed Zwitterionic Peptide as Antifouling Biosensing Interface for Electrochemical Detection of alpha-Fetoprotein. ACS Sens. 2017, 2, 490–494. [Google Scholar] [CrossRef]
- Du, Y.; Lim, B.J.; Li, B.; Jiang, Y.S.; Sessler, J.L.; Ellington, A.D. Reagentless, Ratiometric Electrochemical DNA Sensors with Improved Robustness and Reproducibility. Anal. Chem. 2014, 86, 8010–8016. [Google Scholar] [CrossRef]
- Xiong, E.; Li, Z.; Zhang, X.; Zhou, J.; Yan, X.; Liu, Y.; Chen, J. Triple-Helix Molecular Switch Electrochemical Ratiometric Biosensor for Ultrasensitive Detection of Nucleic Acids. Anal. Chem. 2017, 89, 8830–8835. [Google Scholar] [CrossRef]
- Meng, F.; Chen, X.; Cheng, W.; Hu, W.; Tang, Y.; Miao, P. Ratiometric Electrochemical Sensing Strategy for the Ultrasensitive Detection of Telomerase Activity. Chemelectrochem 2019, 6, 2000–2003. [Google Scholar] [CrossRef]
- Ren, K.; Wu, J.; Yan, F.; Zhang, Y.; Ju, H. Immunoreaction-triggered DNA assembly for one-step sensitive ratiometric electrochemical biosensing of protein biomarker. Biosens. Bioelectron. 2015, 66, 345–349. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H. Ultrathin Two-Dimensional Nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef] [PubMed]
- Eswaraiah, V.; Zeng, Q.; Long, Y.; Liu, Z. Black phosphorus nanosheets: Synthesis, characterization and applications. Small 2016, 12, 3480–3502. [Google Scholar] [CrossRef] [PubMed]
- Mao, N.; Tang, J.; Xie, L.; Wu, J.; Han, B.; Lin, J.; Deng, S.; Ji, W.; Xu, H.; Liu, K.; et al. Optical Anisotropy of Black Phosphorus in the Visible Regime. J. Am. Chem. Soc. 2015, 138, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Mao, N.; Wu, J.; Han, B.; Lin, J.; Tong, L.; Zhang, J. Birefringence-Directed Raman Selection Rules in 2D Black Phosphorus Crystals. Small 2016, 12, 2627–2633. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Kwong, C.W.; Davey, K.; Qiao, S.Z. 2D phosphorene as a water splitting photocatalyst: Fundamentals to applications. Energy Environ. Sci. 2016, 9, 709–728. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Deng, Y.; Peide, D.Y. Semiconducting black phosphorus: Synthesis, transport properties and electronic applications. Chem. Soc. Rev. 2015, 46, 2732–2743. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Kim, J.; Jin, C.; Ye, G.J.; Qiu, D.Y.; da Jornada, F.H.; Shi, Z.; Chen, L.; Zhang, Z.; Yang, F.; et al. Direct observation of the layer-dependent electronic structure in phosphorene. Nat. Nanotechnol. 2016, 12, 21–25. [Google Scholar] [CrossRef] [Green Version]
- Lei, W.; Zhang, T.; Gu, L.; Liu, P.; Rodriguez, J.A.; Liu, G.; Liu, M. Surface-Structure Sensitivity of CeO2 Nanocrystals in Photocatalysis and Enhancing the Reactivity with Nanogold. ACS Catal. 2015, 5, 4385–4393. [Google Scholar] [CrossRef]
- Hu, J.; Guo, Z.; Mcwilliams, P.E.; Darges, J.E.; Druffel, D.L.; Moran, A.M.; Warren, S.C. Band Gap Engineering in a 2D Material for Solar-to-Chemical Energy Conversion. Nano Lett. 2015, 16, 74–79. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Shao, W.; Chen, S.; Xie, J.; Zhang, X.; Wang, J.; Xie, Y. Ultrathin Black Phosphorus Nanosheets for Efficient Singlet Oxygen Generation. J. Am. Chem. Soc. 2015, 137, 11376–11382. [Google Scholar] [CrossRef]
- Ren, X.; Li, Z.; Huang, Z.; Sang, D.; Qiao, H.; Qi, X.; Li, J.; Zhong, J.; Zhang, H. Environmentally Robust Black Phosphorus Nanosheets in Solution: Application for Self-Powered Photodetector. Adv. Funct. Mater. 2017, 27, 1606834. [Google Scholar] [CrossRef]
- Lei, W.; Liu, G.; Zhang, J.; Liu, M. Black phosphorus nanostructures: Recent advances in hybridization, doping and functionalization. Chem. Soc. Rev. 2017, 46, 3492–3509. [Google Scholar] [CrossRef]
- Sun, J.; Lee, H.-W.; Pasta, M.; Yuan, H.; Zheng, G.; Sun, Y.; Li, Y.; Cui, Y. A phosphorene–graphene hybrid material as a high-capacity anode for sodium-ion batteries. Nat. Nanotechnol. 2015, 10, 980–985. [Google Scholar] [CrossRef]
- Ryder, C.R.; Wood, J.D.; Wells, S.A.; Yang, Y.; Jariwala, D.; Marks, T.J.; Schatz, G.C.; Hersam, M.C. Covalent functionalization and passivation of exfoliated black phosphorus via aryl diazonium chemistry. Nat. Chem. 2016, 8, 597–602. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Qiao, X.; Wang, Y.; Sheng, Q.; Yue, T.; Zheng, J.; Zhou, M. Electrostatic assembly of gold nanoparticles on black phosphorus nanosheets for electrochemical aptasensing of patulin. Microchim. Acta 2019, 186, 238. [Google Scholar] [CrossRef]
- Liu, B.; Abbas, A.; Zhou, C. Two-Dimensional Semiconductors: From Materials Preparation to Electronic Applications. Adv. Electron. Mater. 2017, 3, 1700045. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, H.; Shi, Z.; Duan, N.; Fang, C.; Dai, S.; Wang, Z. Aptamer-based fluorescence biosensor for chloramphenicol determination using upconversion nanoparticles. Food Control. 2015, 50, 597–604. [Google Scholar] [CrossRef]
- Wang, J.; Liu, D.; Huang, H.; Yang, N.; Yu, B.; Wen, M.; Wang, X.; Chu, P.K.; Yu, X.-F. In-Plane Black Phosphorus/Dicobalt Phosphide Heterostructure for Efficient Electrocatalysis. Angew. Chem. 2017, 130, 2630–2634. [Google Scholar] [CrossRef]
- Kang, J.; Wood, J.D.; Wells, S.A.; Lee, J.H.; Liu, X.; Chen, K.S.; Hersam, M.C. Solvent exfoliation of electronic-grade, two-dimensional black phosphorus. ACS Nano 2015, 9, 3596–3604. [Google Scholar] [CrossRef] [Green Version]
- Xia, F.; Wang, H.; Jia, Y. Rediscovering black phosphorus as an anisotropic layered material for optoelectronics and electronics. Nat. Commun. 2014, 5, 4458. [Google Scholar] [CrossRef] [Green Version]
- Wood, J.D.; Wells, S.A.; Jariwala, D.; Chen, K.-S.; Cho, E.; Sangwan, V.K.; Liu, X.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Effective Passivation of Exfoliated Black Phosphorus Transistors against Ambient Degradation. Nano Lett. 2014, 14, 6964–6970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, N.B.; Ley, L.; Bullett, D.W. Valence-band structures of phosphorus allotropes. Phys. Rev. B 1983, 27, 7440–7450. [Google Scholar] [CrossRef]
- Brunner, J.; Thüler, M.; Veprek, S.; Wild, R. X-ray photoelectron study of amorphous phosphorus preparedbyplasmachemical transport. Comparison with crystalline polymorphs. J. Phys. Chem. Solids 1979, 40, 967–971. [Google Scholar] [CrossRef]
- Han, Q.; Wang, R.; Xing, B.; Zhang, T.; Khan, M.S.; Wu, D.; Wei, Q. Label-free photoelectrochemical immunoassay for CEA detection based on CdS sensitized WO3@BiOI heterostructure nanocomposite. Biosens. Bioelectron. 2018, 99, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Zhang, M.; Li, B.; Huang, D.; Zeng, G.; Qin, L.; Liu, X.; Yi, H.; Cheng, M.; Li, L.; et al. Fabrication of CuS/BiVO4 (0 4 0) binary heterojunction photocatalysts with enhanced photocatalytic activity for Ciprofloxacin degradation and mechanism insight. Chem. Eng. J. 2018, 358, 891–902. [Google Scholar] [CrossRef]
- Wu, S.; Duan, N.; Zhang, W.; Zhao, S.; Wang, Z. Screening and development of DNA aptamers as capture probes for colorimetric detection of patulin. Anal. Biochem. 2016, 508, 58–64. [Google Scholar] [CrossRef]
- Bagheri, N.; Khataee, A.; Habibi, B.; Hassanzadeh, J. Mimetic Ag nanoparticle/Zn-based MOF nanocomposite (AgNPs@ ZnMOF) capped with molecularly imprinted polymer for the selective detection of patulin. Talanta 2018, 179, 710–718. [Google Scholar] [CrossRef]
- Malysheva, S.V.; Di Mavungu, J.D.; Boonen, J.; De Spiegeleer, B.; Goryacheva, I.Y.; Vanhaecke, L.; De Saeger, S. Improved positive electrospray ionization of patulin by adduct formation: Usefulness in liquid chromatography–tandem mass spectrometry multi-mycotoxin analysis. J. Chromatogr. A 2012, 1270, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Xu, E.; Jin, Z.; Irudayaraj, J. An ultrasensitive aptasensor based on fluorescent resonant energy transfer and exonuclease-assisted target recycling for patulin detection. Food Chem. 2018, 249, 136–142. [Google Scholar] [CrossRef]
- Funari, R.; Della Ventura, B.; Carrieri, R.; Morra, L.; Lahoz, E.; Gesuele, F.; Altucci, C.; Velotta, R. Detection of parathion and patulin by quartz-crystal microbalance functionalized by the photonics immobilization technique. Biosens. Bioelectron. 2015, 67, 224–229. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Fan, Z. Determination of patulin in apple juice using magnetic solid-phase extraction coupled with high-performance liquid chromatography. Food Addit. Contam. Part A 2017, 34, 273–281. [Google Scholar] [CrossRef]
- Zhang, W.; Han, Y.; Chen, X.; Luo, X.; Wang, J.; Yue, T.; Li, Z. Surface molecularly imprinted polymer capped Mn-doped ZnS quantum dots as a phosphorescent nanosensor for detecting patulin in apple juice. Food Chem. 2017, 232, 145–154. [Google Scholar] [CrossRef]









| Methos | Linear Range (ng/mL) | LOD (ng/mL) | Reference |
|---|---|---|---|
| Colorimetric detection | 0.046–24.64 | 0.046 | [46] |
| Florescent detection | 15.4–1540 | 9.24 | [47] |
| Gas chromatography-Mass spectrometry | 64.9–1623 | 5.00 | [48] |
| Fluorescent resonant energy transfer | 0.0092–92.4 | 0.003 | [49] |
| Quartz crystal microbalance immunoassay | 30.8–154 | 21.56 | [50] |
| High performance chromatography | 0.92–400.4 | 0.154 | [51] |
| Phosphorescent nanosensor | 66.2–1001 | 49.28 | [52] |
| Electrochemical detection | 0.0154–15.4 | 0.043 | This work |
| Samples | Background Concentration (μg/mL) | Added Concentration (μg/mL) | Found Concentration (μg/mL, HPLC) | Found Concentration (μg/mL, the Sensor) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|---|---|
| Fresh apple | ND | 0.092 | 0.075 | 0.089 | 96.7 | 3.31 |
| Fresh pear | ND | 0.092 | 0.077 | 0.088 | 95.0 | 2.92 |
| Fresh tomato | ND | 0.092 | 0.088 | 0.094 | 101.7 | 4.05 |
| Infected apple | 0.035 | 0.092 | 0.104 | 0.131 | 103.3 | 4.31 |
| 0.308 | 0.298 | 0.335 | 97.5 | 3.12 | ||
| Infected pear | 0.032 | 0.092 | 0.099 | 0.131 | 106.7 | 4.56 |
| 0.308 | 0.301 | 0.344 | 101.5 | 3.60 | ||
| Infected tomato | 0.071 | 0.092 | 0.113 | 0.159 | 96.7 | 5.14 |
| 0.308 | 0.315 | 0.374 | 98.5 | 3.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Liu, J.; Li, W.; Wei, Y.; Sheng, Q.; Shang, Y. A Dual-Signaling Electrochemical Aptasensor Based on an In-Plane Gold Nanoparticles–Black Phosphorus Heterostructure for the Sensitive Detection of Patulin. Foods 2023, 12, 846. https://doi.org/10.3390/foods12040846
Xu J, Liu J, Li W, Wei Y, Sheng Q, Shang Y. A Dual-Signaling Electrochemical Aptasensor Based on an In-Plane Gold Nanoparticles–Black Phosphorus Heterostructure for the Sensitive Detection of Patulin. Foods. 2023; 12(4):846. https://doi.org/10.3390/foods12040846
Chicago/Turabian StyleXu, Jinqiong, Jianbo Liu, Wuwu Li, Yongsheng Wei, Qinglin Sheng, and Yonghui Shang. 2023. "A Dual-Signaling Electrochemical Aptasensor Based on an In-Plane Gold Nanoparticles–Black Phosphorus Heterostructure for the Sensitive Detection of Patulin" Foods 12, no. 4: 846. https://doi.org/10.3390/foods12040846