Au Nanoparticles Functionalized Covalent-Organic-Framework-Based Electrochemical Sensor for Sensitive Detection of Ractopamine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus and Characterization
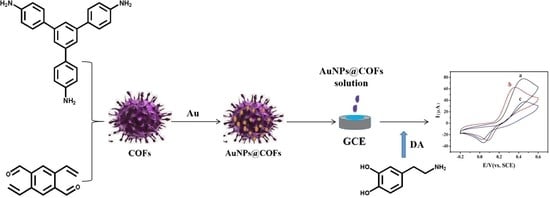
2.3. Preparation of COFs Composite Nanomaterials
2.4. Construction of AuNPs@COFs Nanocomposites Electrochemical Sensor
2.5. Pretreatment of Actual Samples
3. Results and Discussion
3.1. Characterization of COFs Nanomaterials and AuNPs@COFs Nanocomposites
3.2. Electrochemical Performance of AuNPs@COFs Nanocomposites
3.3. Amperometric Detection of Ractopamine
3.4. Selectivity, Stability and Repeatability Analysis of Proposed Sensor
3.5. Detection in Actual Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jing, H.; Ouyang, H.Y.; Li, W.F.; Long, Y. Molten salt synthesis of BCNO nanosheets for the electrochemical detection of clenbuterol. Microchem. J. 2022, 178, 107359. [Google Scholar] [CrossRef]
- Soares, M.H.; Junior, D.T.V.; de Amorim, R.G.; Júnior, R.L.C.; Rocha, G.C.; Bohrer, B.M.; Juárez, M.; de Souza Duarte, M.; Saraiva, A. Effects of feeding ractopamine hydrochloride with or without supplemental betaine on live performance, carcass and meat quality traits, and gene expression of finishing pigs. Meat Sci. 2022, 191, 108851. [Google Scholar] [CrossRef] [PubMed]
- Liu, F. The impact of the Codex Alimentarius Commission ractopamine standard on China’s pork trade. Chin. J. Anim. Husb. 2015, 51, 37–40. [Google Scholar]
- Du, W.; Zhao, G.; Fu, Q.; Sun, M.; Zhou, H.; Chang, C. Combined microextraction by packed sorbent and high-performance liquid chromatography-utraviolet detection for rapid analysis of ractopamine in porcine muscle and urine samples. Food Chem. 2014, 145, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Decheng, S.; Peilong, W.; Yang, L.; Ruiguo, W.; Xia, F.; Xiaoou, S. Evaluation of β-agonists in blood meal:Validation of determination method and potential pathway for reentry into the environment. J. Chromatogr. A 2019, 1612, 460624. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.E.; Peng, Z.C. Comparison and application of three extraction methods in detection of receptor agonist residues in pig liver. Modern Food 2019, 3, 182–184. [Google Scholar]
- SN/T 4818-2017; Determination of Ractopamine, Salbutamol and Clenbuterol Hydrochloride in Import and Export Edible Animals Enzyme-Linked Immunosorbent Assay. Chinese Standard. AQSIQ: Beijing, China, 2017.
- Zhang, Y.; Wang, F.X.; Fang, L.; Wang, S.; Fang, G. Rapid determination of ractopamine residues in edible animal products by enzyme-linked immunosorbent assay: Development and investigation of matrix effects. Biomed Res. Int. 2014, 2009, 269–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tefera, M.; Tessema, M.; Admassie, S.; Wubet, W. Voltammetric determination of uric acid using multiwall carbon nanotubes coated-poly (4-amino-3-hydroxy naphthalene sulfonic acid) modified glassy carbon electrode. Heliyon 2021, 7, e07575. [Google Scholar] [CrossRef]
- Yan, X.; Song, Y.; Liu, J.; Zhou, N.; Zhang, C.; He, L.; Zhang, Z.; Liu, Z. Two-dimensional porphyrin-based covalent organic framework: A novel platform for sensitive epidermal growth factor receptor and living cancer cell detection. Biosens. Bioelectron. 2019, 126, 734–742. [Google Scholar] [CrossRef]
- Yang, Y.X.; Shen, Y.; Wang, L.; Song, Y.; Wang, L. Three-dimensional porous carbon/covalent-organic framework films integrated electrode for electrochemical sensors. J. Electroanal. Chem. 2019, 855, 113590. [Google Scholar] [CrossRef]
- Liang, H.; Xu, H.T.; Zhao, Y.T.; Zheng, J.; Zhao, H.; Li, G.; Li, C.P. Ultrasensitive electrochemical sensor for prostate specific antigen detection with a phosphorene platform and magnetic covalent organic framework signal amplifier. Biosens. Bioelectron. 2019, 144, 111691. [Google Scholar] [CrossRef] [PubMed]
- Kordasht, H.K.; Saadati, A.; Hasanzadeh, M. A flexible paper based electrochemical portable biosensor towards recognition of ractopamine as animal feed additive: Low cost diagnostic tool towards food analysis using aptasensor technology. Food Chem. 2022, 373, 131411. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, R.; Mahsa, G.; Faezeh, S.F. Fabrication of an electrochemical biodevice for ractopamine detection under a strategy of a double recognition of the aptamer/molecular imprinting polymer. Bioelectrochemistry 2021, 138, 107722. [Google Scholar]
- Cao, L.P.; Ding, Q.; Liu, M.H.; Lin, H.; Yang, D.P. Biochar-supported Cu2+/Cu+ composite as an electrochemical ultrasensitive interface for ractopamine detection. ACS Appl. Bio Mater. 2021, 4, 1424–1431. [Google Scholar] [CrossRef]
- Murugan, K.; Asit, K.P.; Wang, Y.H.; Liu, X.; He, J.H.; Chung, R.J. Titanium nanoparticle anchored functionalized MWCNTs for electrochemical detection of ractopamine in porcine samples with ultrahigh sensitivity. Food Chem. 2022, 378, 132083. [Google Scholar]
- Zhou, Y.; Wang, T.T.; Yan, L.T. A sensitive electrochemical analysis method of ractopamine based on Janus particles. Chin. J. Anal. Chem. 2022, 50, 1872–2040. [Google Scholar] [CrossRef]
- Jin, E.; Geng, K.Y.; Lee, K.H.; Jiang, W.; Li, J.; Jiang, Q.; Irle, S.; Jiang, D. Topology-templated synthesis of crystalline porous covalent organic frameworks. Angew. Chem. 2020, 132, 12260–12267. [Google Scholar] [CrossRef]
- Tan, K.T.; Tao, S.; Huang, N.; Jiang, D. Water cluster in hydrophobic crystalline porous covalent organic frameworks. Nat. Commun. 2021, 12, 6747. [Google Scholar] [CrossRef]
- Liu, X.L.; Li, J.; Gui, B.; Lin, G.; Fu, Q.; Yin, S.; Liu, X.; Sun, J.; Wang, C. A crystalline three-dimensional covalent organic framework with flexible building blocks. J. Am. Chem. Soc. 2021, 143, 2123–2129. [Google Scholar] [CrossRef]
- Zhao, H.; Xie, W.; Zhang, R.L.; Wang, X.D.; Liu, H.F.; Li, J.; Sha, T.; Guo, X.S.; Sun, Q.M.; Zhang, Y.P.; et al. Electrochemical sensor for human norovirus based on covalent organic framework/pillararene heterosupramolecular nanocomposites. Talanta 2022, 237, 122896. [Google Scholar] [CrossRef]
- Sun, Y.; He, J.; Waterhouse, G.I.N.; Xu, L.; Zhang, H.; Qiao, X.; Xu, Z. A selective molecularly imprinted electrochemical sensor with GO@COF signal amplification for the simultaneous determination of sulfadiazine and acetaminophen. Sens. Actuators B Chem. 2019, 300, 126993. [Google Scholar] [CrossRef]
- Wang, J.; Feng, Y.Q.; Zhang, B. MOF-COF hybrid frameworks materials. Prog. Chem. 2022, 34, 1308–1320. [Google Scholar]
- Li, Y.; Guo, X.H.; Li, X.F.; Zhang, M.; Jia, Z.; Deng, Y.; Tian, Y.; Li, S.; Ma, L. Redox-active two-dimensional covalent organic frameworks (COFs) for selective reductive separation of valence-variable, redox-sensitive and long-lived radionuclides. Angew. Chem. 2020, 132, 4197–4204. [Google Scholar] [CrossRef]
- Tian, Y.Y.; Lu, Q.P.; Guo, X.X.; Wang, S.Y.; Gao, Y.; Wang, L.H. Au nanoparticles deposited on ultrathin two-dimensional covalent organic framework nanosheets for in vitro and intracellular sensing. Nanoscale 2020, 12, 7776–7781. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, N.; Wang, C.; Gao, W.; Chen, M.; Liu, N.; Duan, J.; Hou, B. Electrochemical detection of hydroquinone and catechol with covalent organic framework modified carbon paste electrode. J. Electroanal. Chem. 2020, 877, 114530. [Google Scholar] [CrossRef]
- Jin, F.Y.; Nguyen, H.L.; Zhong, Z.Y.; Han, X.; Zhu, C.; Pei, X.; Ma, Y.; Yaghi, O.M. Entanglement of square nets in covalent organic frameworks. J. Am. Chem. Soc. 2022, 144, 1539–1544. [Google Scholar] [CrossRef]
- Ma, W.; Zheng, Q.; He, Y.; Li, G.; Guo, W.; Lin, Z.; Zhang, L. Size-controllable synthesis of uniform spherical covalent organic frameworks at room temperature for highly efficient and selective enrichment of hydrophobic peptides. J. Am. Chem. Soc. 2019, 141, 18271–18277. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Qian, H.L.; Zhao, X.; Yang, C.X.; Yan, X.P. Fabrication of a covalent organic framework and its gold nanoparticle hybrids as stable mimetic peroxidase for sensitive and selective colorimetric detection of mercury in water samples. Talanta 2019, 204, 224–228. [Google Scholar] [CrossRef]
- Zhang, X.L.; Luo, Y.J.; Jiang, J.; Lu, Y.; Feng, N. Determination of five β2-agonist residues in pork by ultra-high performance liquid chromatography-tandem mass spectrometry with molecularly imprinted solid phase extraction. Chin. J. Chromatogr. 2015, 33, 838. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Huang, W.; Wang, Y.; Hu, X. A novel AuNPs-doped COFs composite as electrochemical probe for chlorogenic acid detection with enhanced sensitivity and stability. Sens. Actuators B Chem. 2018, 276, 362–369. [Google Scholar] [CrossRef]
- Klingler, R.J.; Kochi, J.K. Electron-transfer kinetics from cyclic voltammetry. Quantitative description of electrochemical reversibility. J. Phys. Chem. 1981, 85, 1731–1741. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Q.; Guan, Q.M.; Wu, J.; Li, H.N.; Yan, J.J. Enhanced direct electrochemistry of glucose oxidase and biosensing for glucose via synergy effect of graphene and CdS nanocrystals. Biosens. Bioelectron. 2011, 26, 2252–2257. [Google Scholar] [CrossRef] [PubMed]
- Moru, S.; Kumar, V.S.; Kummari, S.; Yugender Goud, K. A disposable screen printed electrodes with hexagonal Ni(OH)2 nanoplates embedded chitosan layer for the detection of depression biomarker. Micromachines 2023, 14, 146. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.C.; Hong, C.P.; Chen, S.M. Simultaneous determination for toxic ractopamine and salbutamol in pork sample using hybrid carbon nanotubes. Sens. Actuators B Chem. 2013, 177, 428–436. [Google Scholar] [CrossRef]
- Kong, L.J.; Pan, M.F.; Fang, G.Z.; He, X.L.; Yang, Y.K.; Dai, J.; Wang, S. Molecularly imprinted quartz crystal microbalance sensor based on poly(o-aminothiophenol) membrane and Au nanoparticles for ractopamine determination. Biosens. Bioelectron. 2014, 51, 286–292. [Google Scholar] [CrossRef]
- Li, J.J.; Bo, X.J. Laser-enabled flexible electrochemical sensor on finger for fast food security detection. J. Hazard. Mater. 2022, 423, 127014. [Google Scholar] [CrossRef]
- Luo, S.X.; Wu, Y.H.; Gou, H.; Liu, Y. A novel electrochemical sensor for the analysis of salbutamol in pork samples by using NiFe2O4 nanoparticles modified glassy carbon electrode. Adv. Mater. Res. 2013, 850–851, 1279–1282. [Google Scholar] [CrossRef]
- Wang, Q.W.; Wang, M.; Zhang, N.; Huang, X.; Wang, X.; Wang, S. A novel electrochemical sensor based on MoS2 electrospun nanofibers and polyoxometalate composite for the simultaneous detection of ractopamine and clenbuterol. Microchem. J. 2023, 108434. [Google Scholar] [CrossRef]










| Electrode * | Linear Range (μmol/L) | Limit of Detection (LOD) (μmol/L) | Reference |
|---|---|---|---|
| SMWCNT-NF/ITO | 0.3–33.3 | 0.1 | [35] |
| PoAT-AuNP-Au/SCE | 2.5–150 | 1.17 | [36] |
| FLG-PI/FGE | 5–80 | 1.29 | [37] |
| 10–200 | 2.7 | ||
| 25–250 | 7.81 | ||
| NiFe2O4/GCE | 2–60 | 1 | [38] |
| Nafion/HPMo/MoS2/PDDA/GCE | 1–70 | 0.06 | [39] |
| AuNPs@COFs/GCE | 1.20–280280–1600 | 0.12 | This work |
| Sample | Ractopamine Added (mmol/L) | HPLC Determination Concentration (mmol/L) | Electrochemical Sensor | ||
|---|---|---|---|---|---|
| Determination Concentration (mmol/L) | Relative Standard Deviation (RSD) | Recovery Rate | |||
| Sample 1 | 0.05 | 0.08 | 0.08 | 2.51% | 98.0% |
| 1.00 | 1.06 | 0.94 | 5.12% | 95.2% | |
| 2.00 | 2.10 | 2.08 | 3.13% | 101.0% | |
| Sample 2 | 0.1 | 0.16 | 0.15 | 4.01% | 97.5% |
| 1.00 | 1.10 | 1.07 | 3.73% | 98.3% | |
| 2.00 | 2.09 | 2.11 | 2.13% | 108.1% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Yang, R.; He, J.; Zhang, Y.; Yuan, Y.; Yue, T.; Sheng, Q. Au Nanoparticles Functionalized Covalent-Organic-Framework-Based Electrochemical Sensor for Sensitive Detection of Ractopamine. Foods 2023, 12, 842. https://doi.org/10.3390/foods12040842
Yang S, Yang R, He J, Zhang Y, Yuan Y, Yue T, Sheng Q. Au Nanoparticles Functionalized Covalent-Organic-Framework-Based Electrochemical Sensor for Sensitive Detection of Ractopamine. Foods. 2023; 12(4):842. https://doi.org/10.3390/foods12040842
Chicago/Turabian StyleYang, Shuying, Ruixi Yang, Jingyi He, Yu Zhang, Yahong Yuan, Tianli Yue, and Qinglin Sheng. 2023. "Au Nanoparticles Functionalized Covalent-Organic-Framework-Based Electrochemical Sensor for Sensitive Detection of Ractopamine" Foods 12, no. 4: 842. https://doi.org/10.3390/foods12040842